Introduction
The assessment of visual memory is one way to examine memory performance. The Rey Visual Design Learning Test (RVDLT) is an elementary motion, colour- and word-independent visual memory test that consists of 15 abstract, black- and-white-lined, straightforward, two-dimensional figures Reference Spreen and Strauss(1). Subjects are required to memorise the figures in five stages. The number of recalled figures counts for the performance.
Anatomical disconnection between brain regions that underpin different aspects of memory function may produce different patterns of memory disturbance (Reference Begré, Federspiel, Kiefer, Schroth, Strik and Dierks2–Reference Dimond, Scammell, Brouwers and Weeks7). Diffusion tensor imaging (DTI) is a relatively novel means of assessing tissue structure providing information about white matter structure. DTI measures diffusion of water molecules in three dimensions. Diffusion of water perpendicular to the direction of the axons is restricted by the myelin sheath and cell membrane such as that the diffusion will be greater along the length of the axon than perpendicular to the axon. Thus, DTI measures diffusion-driven displacements of molecules during their random path along axonal fibres, expressed as fractional anisotropy (FA) or intervoxel coherence (IC) ranging from 0 (isotropic medium) to 1 (fully anisotropic medium). FA is a measure that quantifies the degree to which diffusion differs in the three dimensions. IC considers the degree of collinearity between the diffusion tensor of the reference voxel and the adjacent voxels, and, moreover, yields a better signal-to-noise ratio than the commonly used FA Reference Pierpaoli and Basser(8,Reference Skare, Li, Nordell and Ingvar9). Hence, based on the determination of the similarity of orientation of adjacent voxels, IC reflects a measure of connectivity, expressing fibre coherence at the voxel level with a spatial sampling limited by voxel size. The relation between diffusivity of water molecules in human tissue expressed by anisotropy indices and clinical symptoms was verified post-mortem. Myelin content and axonal density strongly correlated with diffusion anisotropy Reference Mottershead, Schmierer and Clemence(10).
A relationship between anisotropy measured by DTI and cognitive function has been shown in healthy people as well as in patients. Individual differences in reaction time performance of a visual choice-paced reaction time test (CRT) in healthy subjects were associated with variations in the white matter underlying the visuospatial attention network; this suggests that increased anisotropy would be a manifestation of faster nerve conduction velocity leading to faster RT Reference Tuch, Salat, Wisco, Zaleta, Hevelone and Rosas(11). White matter anisotropy decreased linearly with increasing age and directly correlated with executive function assessed by the Trail Making Test Reference O’Sullivan, Jones, Summers, Morris, Williams and Markus(12). Reading scores in reading-impaired adults and their control group were significantly directly correlated with white matter diffusion anisotropy Reference Klingberg, Hedehus and Temple(13). Alcoholics had lower regional FA and IC than controls, and a positive association between anisotropy and working memory and attention was also found in alcoholics compared with controls Reference Pfefferbaum, Sullivan, Hedehus, Lim, Adalsteinsson and Moseley(14). In addition, there was a relationship between FA values and mini-mental state examination (MMSE) scores in Alzheimer’s disease Reference Sun, Du, Zhang and Chen(15). Verbal memory performance directly correlated significantly with posterior cingulate bundle anisotropy in Alzheimer’s disease and minimal brain impairment and in healthy subjects Reference Fellgiebel, Muller and Wille(16). FA was positively associated with verbal declarative memory in patients suffering from schizophrenia Reference Lim, Ardekani, Nierenberg, Butler, Javitt and Hoptman(17). FA values for the splenium of corpus callosum were significantly reduced in patients infected with human immunodeficiency virus and correlated with dementia severity Reference Wu, Storey, Cohen, Epstein, Edelman and Ragin(18). Taken together, a positive correlation between anisotropy and most cognitive measures was shown suggesting that higher cognitive performance is related to biological factors (myelination, fibre density and tissue organisation) that underpin measures such as FA and IC.
The previous findings on a relationship between white matter structure detected by diffusion tensor imaging and cognitive function in neuropsychiatric diseases and healthy people imply that visual memory performance may be associated with white matter structure (Reference Tuch, Salat, Wisco, Zaleta, Hevelone and Rosas11,Reference Peters19,Reference Oppenheim, Rodrigo and Poupon20). As far as we know, however, there is only one study examining visual memory and white matter anisotropy in healthy subjects Reference Begre, Frommer, Von Kanel, Kiefer and Federspiel(21). In the latter study, Begré et al. found a structural difference in white matter between healthy subjects performing low vs. high on the RVDLT, observation of which provided in vivo evidence for the contribution of white matter structure to visual memory.
The present study was designed to test the hypothesis whether interindividual differences in visual memory performance (expressed as number of recalled figures in the RVDLT) may be related to interindividual differences in white matter connectivity (expressed as IC). Based on previous results, we specifically hypothesised that visual memory performance would directly be correlated with IC in the dorsal hippocampal commissure Reference Gloor, Salanova, Olivier and Quesney(22,Reference Ishai, Haxby and Ungerleider23), the corpus callosum (Reference Boldrini, Zanella, Cantagallo and Basaglia24–Reference Alsaadi, Binder, Lazar, Doorani and Mohr26), the posterior cingulate Reference Berthoz(27), the superior longitudinal fascicle Reference Tamura, Takahashi, Kurihara, Yamada, Hatazawa and Okudera(28) and the internal capsule Reference Nolte(29) supporting the notion that all of these brain areas would be involved in visual memory processing.
Material and methods
Subjects
Fourteen right-handed healthy volunteers with no history of major medical, neurological or psychiatric disease were recruited. The study protocol was approved by the local ethics committee (Table 1) and all subjects provided written informed consent. Subjects were instructed to relax and to keep their head still in the magnetic resonance imaging (MRI) scanner. Head motion was minimised with restraining foam pads.
Table 1 Subject characteristics

* Not significant considering age, intelligence, and performance (p < 0.05, Mann–Whitney).
Methods
Performance of the RVDLT. The examiner presented 15 geometric stimulus cards (10 × 7 cm) in a row for 2 s each. After all cards had been presented, the subject was asked to draw all the designs he or she could recall. For the first recall, the subject had 60 s to draw the designs before the examiner removed the recall sheet. This procedure was repeated until five successive trials with different recall sheets had been completed. For the last four trials, the time allowed for the drawings was 90 s. Eventually, 45 min after completion of the last trial, subjects were asked to redraw from memory as many of the 15 figures as possible. The number of accurately drawn figures was added up to a performance score ranging from 0 to 15 points.
MRI and DTI recording. MRI was performed on a 1.5-Tesla standard clinical MRI scanner (Siemens Vision, Erlangen, Germany) using the standard radiofrequency head coil. A high-resolution three-dimensional (3-D) data set covering the whole brain was collected for each subject through a 3-D magnetisation prepared rapid acquisition gradient echo (MP-RAGE) sequence. Totally 192 slices were accumulated [repetition time (TR) = 6 s, echo time (TE) = 95 ms, matrix size = 256 × 256 voxels, field of view (FOV) = 256 mm, voxel dimension = 1.0 × 1.0 × 1.0 mm]. For diffusion-weighted imaging, a single shot spin-echo echo-planar imaging (SE-EPI) sequence was acquired in the same session. The imaging parameters of the single-shot SE-EPI sequence were defined as follows: matrix size = 96 × 128 voxels, TE = 112 ms, FOV = 240 × 240 mm2, slice thickness = 5 mm, 12 axial continuous slices, TR = 3 s, pixel bandwidth = 125 kHz, and use of standard head coil and head neck standard shimming. Gradient amplitudes and duration were set to enable detection of tissue-dependent diffusion coefficients by the signal attenuation: G = 22 mT/m, duration TE = 0 ms, intergradient time interval = 40 ms. The diffusion sensitising gradients were applied simultaneously on two axes around the 180° pulse at b = 1800 s/mm2/axis along six noncollinear directions: (Gx, Gy, Gz) = [(1, 0, 1), (1, 0, −1), (0, 1, 1), (0, 1, −1), (1, 1, 0), (−1, 1, 0)]. This gradient scheme was chosen to minimise acquisition time despite suggestions that the optimal gradient scheme may include more than six gradient directions Reference Jones, Horsfield and Simmons(30,Reference Jones, Symms, Cercignani and Howard31). Additionally, one image was acquired with no gradients applied. The calculation and diagonalisation of the diffusion tensor were based on the multivariate regression approach Reference Basser and Pierpaoli(32). Eddy current corrections were included. Six independent elements of the diffusion tensor were extracted Reference Basser and Pierpaoli(33). Eigenvalues (magnitude) and eigenvectors (direction) were determined for each voxel, and the IC maps were constructed using the average of the angle between the eigenvector of the largest eigenvalue of a given voxel and its neighbours that represents the extent to which the vectors point the same direction and are, therefore, coherent Reference Pierpaoli and Basser(8). For each subject, these maps were manually co-registered with the 3-D anatomical maps and, thereafter, spatially normalised in the Talairach space Reference Talairach and Tournoux(34) by affine transformation implemented in the BrainVoyager software package. During this co-registration, the voxel dimension of the IC maps was interpolated to 1.0 × 1.0 1.0 mm.
Statistical analysis
Analyses of the images were blinded to visual memory performance. Images were smoothed using a Gaussian filter with a full width at half maximum (FWHM) of 7.5 mm. Automatic segmentation of the 3-D anatomical images (MP-RAGE) for each subject yielded individual probability maps (p < 0.01) for grey and white matter (BrainVoyager QX 1.7; Brain Innovation, Maastricht, the Netherlands). The individual 3-D white matter maps were used to compute the largest possible 3-D white matter template (Fig. 1).

Fig. 1 Representative axial slice in Talairach space of one subject (left), its segmented white matter map (middle), and the largest possible white matter mask including all white matter maps of all subjects (right). To test for correlation of IC values with performance of each subject in the RVDLT, the 3-D segmented white matter maps were used to construct the largest possible white matter mask.
In a first step, voxelwise analyses of the IC values were conducted using linear regression analyses to obtain the pattern of correlation with the performance of each subject in the RVDLT. This correlation pattern was computed for DTI voxels within the 3-D white matter template only. The estimation of the correlation was performed using MATLAB programs (MATLAB version 6, release 13; The MathWorks, Inc., Natick, MA, USA). Areas of significant correlation were identified at the cluster level for the threshold p < 0.01. Regions of interest based on correlation clusters were generated by using BrainVoyager. To identify volume-corrected regions, clusters were defined as 60 or more neighbouring voxels (60 mm3) (p < 0.05). For each cluster, IC values in the clusters were averaged and tabulated, and Talairach coordinates Reference Talairach and Tournoux(34) of the centres of gravity were noted. Clusters were assigned to the underlying white matter using 3-D anatomical data. Location in the brain was selected by both a macroscopic Reference Nowinsky, Bryan and Rhaghavan(35) and a microscopic atlas Reference May(36), both indicating Talairach coordinates.
In a second step, these significant clusters were further analysed using a general linear model (GLM) that allows population inference. We assumed in this model that RVDLT was the only predictor for IC response. To test for non-normality of the residuals, the Shapiro–Wilk test was computed for each voxel of each cluster. With the Shapiro–Wilk test, the null hypothesis is that residuals follow a normal distribution, i.e. if the p-value is greater than alpha value of 0.05, the null hypothesis will not be rejected Reference Jones, Symms, Cercignani and Howard(31). Finally, the explained variances of the GLM model were expressed by the R-Square value.
Results
There were no gender differences in terms of age, intelligence, visual memory performance and IC (non-parametric; p < 0.05). IQ was strongly related to test performance suggesting that higher estimates of intelligence were associated with better performance in visual memory (Pearson’s r = 0.72, df = 12, p < 0.004) (Table 1).
Eleven clusters showed a significant and direct relationship with visual memory test performance (Table 2). Memory performance correlated positively with white matter structure in left and right dorsal hippocampal commissure, left and right posterior cingulate, right callosal splenium, left and right superior longitudinal fascicle, right medial orbitofrontal region, left anterior cingulate, and left and right anterior limb of internal capsule (Fig. 2).
Table 2 Clusters with significant correlation between IC and visual memory performance (p < 0.05)

ACC, anterior cingulate cortex; C, capsule; CC, corpus callosum; HC, hippocampus; PCC, posterior cingulate cortex; SLF, superior longitudinal fascicle.
* Positive x-coordinates are in the right, negative x-coordinates in the left hemisphere.
† T-value of ±random effect GLM with model assuming that RVDLT was the only predictor for IC response.
‡ p = exact level of significance of correlation.
§ Explained Variance derived from R-Square in GLM.
¶ Shapiro–Wilk test for non-normality of the residuals.
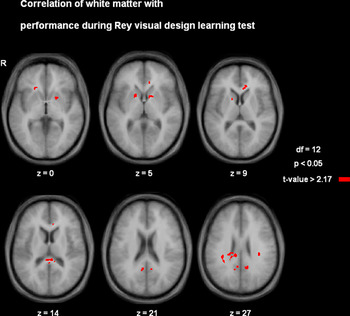
Fig. 2 Axial slices of a representative subject showing the locations of statistically significant correlations of white matter with performance (p < 0.05). Numbers assigned to each slice indicate the z-coordinate in Talairach space.
The correlations between IC cluster values and visual memory test performance maintained significance with IQ as a covariate (all p < 0.05).
Discussion
In the present study, we show in 14 healthy subjects that visual memory measured by the RVDLT is related to physiological properties in white matter structure, suggesting that individual differences in visual memory performance may be because of differences in white matter connectivity. In a voxel-based linear regression analysis, we assessed the relationship between brain function and white matter connectivity by means of the intervoxel anisotropy index (IC) and with visual memory performance. We observed that performance correlated with white matter structure of the left and right dorsal hippocampal commissure, the left and right posterior cingulate, the right callosal splenium, the left and right superior longitudinal fascicle, the right medial orbitofrontal region, the left anterior cingulate and the left and right anterior limb of internal capsule.
Most commonly used visual learning test paradigms imply 3-D, colour, motion or word-dependent brain operations. We chose the RVDLT as a straightforward visual learning paradigm, using elementary, geometric, black-and-white-lined figures. This design avoids a significant contribution from as many additional higher order visual brain functions as possible to visual performance.
Supporting our hypothesis and corroborating previous studies showing a relationship between anisotropy measured by DTI and memory performance in memory-affected patients (Reference Begré, Federspiel, Kiefer, Schroth, Strik and Dierks2,Reference O’Sullivan, Jones, Summers, Morris, Williams and Markus12,Reference Klingberg, Hedehus and Temple13,Reference Begre and Koenig37–Reference Muller, Greverus and Dellani40), and in healthy subjects Reference Begré, Frommer, Von Kanel, Kiefer and Federspiel(41), visual memory performance was positively correlated with white matter structure in 11 definite brain regions. This observation suggests that the less connectivity in the white matter bundles in these regions, the poorer the memory performance on the one hand, and that the more connectivity structure shown in the white matter bundles in these clusters, the better the memory performance on the other. Therefore, we suggest that, in brain regions where memory performance is correlated with IC, the white matter bundles support processing RVDLT. The fact that we did not find any statistical association with RVDLT performance in the clusters representing correlation of IC with RVDLT performance (data not shown) in a cancellation test requiring visual scanning (d2 test) Reference Spreen and Strauss(1) supports our interpretation. We therefore may state that the clusters of white matter regions were mainly independent of processing of visual scanning but instead related to neuron bundles involved in the task. Hence, we may assume that significant voxel clusters are structural correlates of specifically visual memory capacity. Incomplete myelination in general, associated with white matter changes, may lead to slower information processing, aberrant neuronal signalling, and, therefore, lower task performance. Accordingly, the more efficient the regional myelination, the faster the information processing on this site, and, eventually, the higher the task performance (Reference Spreen and Strauss1,Reference Wu, Storey, Cohen, Epstein, Edelman and Ragin18,Reference Begré, Federspiel, Kiefer, Schroth, Strik and Dierks42). In other words, individual structural differences may lead to different functionality (Reference Begré, Federspiel, Kiefer, Schroth, Strik and Dierks2,Reference Begré, Federspiel, Kiefer, Schroth, Dierks and Strik5,Reference Begre and Koenig37,Reference Begré, Frommer, Von Kanel, Kiefer and Federspiel41,Reference Ridler, Veijola and Tanskanen43–Reference Ho, Andreasen, Nopoulos, Arndt, Magnotta and Flaum45). Corroborating the previous literature about anisotropy in neuropsychiatric diseases and healthy people, we detected a positive association between anisotropy values and brain function (Reference Begré, Federspiel, Kiefer, Schroth, Strik and Dierks2,Reference Wu, Storey, Cohen, Epstein, Edelman and Ragin18–20,Reference Begré, Frommer, Von Kanel, Kiefer and Federspiel41).
The finding of clusters was expected within white matter of the dorsal hippocampal commissure, the corpus callosum, the posterior cingulate, the superior longitudinal fascicle and the internal capsule. A potentially important cluster was found in the dorsal hippocampal commissure where fibres travel between the splenium and the hippocampal formation. Based on previous studies in humans Reference Gloor, Salanova, Olivier and Quesney(22,Reference Palmini, Gloor and Jones-Gotman46), the dorsal hippocampal commissure is supposed to be important for memory function. The hippocampus is activated during retrieval of mental images Reference Ishai, Haxby and Ungerleider(23). The posterior part of the corpus callosum plays a predominant role in the right–left transfer of spatial information guiding constructional performance Reference Ishai, Haxby and Ungerleider(23). As previously shown, impairment of the posterior part of the corpus callosum leads to derogation of human visual memory Reference Hasegawa(25,Reference Alsaadi, Binder, Lazar, Doorani and Mohr26). The posterior cingulate cortex was suggested to support visual recall performance Reference Berthoz(27). An interplay between different neurofunctional systems is important in memory and learning Reference Poldrack and Rodriguez(47,Reference Todd and Marois48). The major association pathway that links the frontal lobe with the parietal cortex is the superior longitudinal fascicle Reference Tamura, Takahashi, Kurihara, Yamada, Hatazawa and Okudera(28). Almost all neural traffic to and from the cerebral cortex passes through the internal capsule Reference Nolte(29).
Furthermore, we found two clusters in the orbitofrontal region and the anterior cingulate, respectively. The orbitofrontal region plays an important role in encoding abstract visual information Reference Frey and Petrides(49). The anterior cingulate cortex seems to be involved if an effort is needed to carry out a task such as early learning and problem solving Reference Allman, Hakeem, Erwin, Nimchinsky and Hof(50), is implicated in reinforcement-guided decision making and error detection Reference Rushworth, Behrens, Rudebeck and Walton(51) and is responsible for rendering new memories permanent Reference Wiltgen, Brown, Talton and Silva(52).
We mention three important limitations of our findings. Firstly, even if acquisition time can be reduced by applying a DTI sequence with a minimum of tensor directions, other advanced acquisition techniques might improve accuracy of measurements. Therefore, future studies should further enhance signal-to-noise ratio by image averaging of a sequence with more tensor directions. Secondly, the physical mechanisms involved in the diffusion anisotropy in white matter are not fully understood. Some authors feel that the diffusion barriers presented by the cell membrane and myelin sheath play a significant role Reference Beaulieu(53). However, IC provides only an indirect maker of white matter structural properties. Thirdly, although there is good clinical and experimental evidence for an association between DTI and cognitive performance in healthy individuals and in patient populations Reference Begré, Federspiel, Kiefer, Schroth, Strik and Dierks(2,Reference Grieve, Williams, Paul, Clark and Gordon54), the a priori assumption that the diffusion of water molecules along axonal bundles can be used as a measure of neural connectivity is, as yet, unproven. Therefore, whether differences in IC between subjects in our study relate to interconnections between neural networks in individuals with different visual learning skills, remains open.
Bearing these limitations in mind, our study suggests in vivo evidence for a contribution of white matter physiological properties to the recall of visual memory in healthy people. DTI could be a promising tool to help to better understand the interconnecting neurofunctional network of visual processing and its impact on clinical behaviour. Based on our findings, further studies combining DTI fibre tracking with functional methods seem a guarantee to learn more about behavioural consequences of structural brain conditions.
Acknowledgements
We thank Regula Schweizer for performing the magnetic resonance imaging measurements and Annette Kocher for proofreading the manuscript.






