Summations
-
∙ Prenatal alcohol exposure results in smaller total brain volume, specific grey and white matter and cortical regions as well as abnormalities in neurochemistry and functional connectivity.
-
∙ There is a gap in the literature of neuroimaging studies in alcohol-exposed children under 5 years of age across all the modalities of neuroimaging.
-
∙ The dynamic nature of brain maturation calls for the prioritisation of studies that include a longitudinal approach to understanding the effects of alcohol exposure on the developing human brain.
Considerations
-
∙ Differing methodologies make inter-study comparisons difficult.
-
∙ Reporting of structural or microstructural alterations in single regions of interest (ROI) has resulted in the effects on specific regions being very well-documented at the expense of a more exploratory approach.
-
∙ Controlling for polysubstance abuse, environmental factors and age is required to assess consistency of results and inter-study comparisons.
Introduction
The term foetal alcohol syndrome (FAS) was only formalised in the 1970s when Jones and Smith described abnormalities and disabilities in children born to mothers with alcoholism (Reference Jones and Smith1,Reference May, Gossage and White‐Country2). The World Health Organisation’s (3) global status report on alcohol and health states that harmful alcohol usage ranks in the top five contributors to disease, disability and mortality. FAS is further recognised as one of the major disease and disability categories within alcohol-related disorders (Reference Lupton, Burd and Harwood4–Reference Navarro, Doran and Shakeshaft6). Studies indicate that the global prevalence of FAS ranges between two and seven per 1000 births, and foetal alcohol spectrum disorders (FASD) between 20 and 50 per 1000 births (Reference May, Blankenship and Marais7–Reference May, Gossage and Kalberg9), with the highest rates in South Africa (Reference Viljoen, Gossage and Brooke10). However, May et al. (Reference May, Gossage and Marais11–Reference May, Baete and Russo14) argue that the current prevalence estimates of FASD represent an underestimate, and FASD represents a much larger public health issue than what has been previously recognised, especially in emerging economies such as South Africa.
FASD animal studies using MRI modalities demonstrate a spectrum of changes in the developing brain structure (Reference O’Leary-Moore, Parnell and Lipinski15). Findings in rodent and mouse FASD models include reports of enlarged ventricles (Reference Mattson, Riley and Jernigan16), diffuse reductions in brain volumes, including whole brain volume (Reference O’Leary-Moore, Parnell and Godin17–Reference Leigland, Ford, Lerch and Kroenke19), cortical grey matter volume (Reference Leigland, Ford, Lerch and Kroenke19), caudate-putamen volume (Reference Mattson, Riley and Jernigan16), hippocampal volume (Reference Parnell, O’Leary-Moore and Godin20) and cerebellar volume (Reference Parnell, O’Leary-Moore and Godin20), whereas the pituitary and septal regions of the brain showed increased volumes (Reference Parnell, O’Leary-Moore and Godin20). Lipinski et al. (Reference Lipinski, Hammond and O’Leary-Moore21) have described the correlation between dysmorphology and brain abnormalities in mice models with prenatal alcohol exposure at different stages of gestation. A single rat FASD diffusion tensor imaging (DTI) study showed increased fractional anisotropy (FA) in cortical tissue (Reference Leigland, Budde, Cornea and Kroenke22). In addition, a single spectroscopy FASD rat study has shown that, postnatal day 4–9, neonatal alcohol exposure results in reduced N-acetyl aspartate (NAA) and taurine in the striatum and cerebellum and increased myoinositol in the cerebellum (Reference O’Leary-Moore, McMechan, Galloway and Hannigan23).
Early investigations into the effects of alcohol on the structure of the human brain have reported on autopsies of children who had died in infancy, although likely to have been on the extreme severe end of the spectrum of alcohol-exposure effects. Findings included microcephaly (most consistently), but also agenesis of the corpus callosum, ventriculomegaly, a small cerebellum as well as a few further malformations due to neuronal and glial migration abnormalities (Reference Jones, Smith, Ulleland and Streissguth24,Reference Coulter, Leech, Schaefer, Scheithauer and Brumback25). Advances in neuroimaging methods over the last decade have allowed researchers to study structural, metabolic and functional abnormalities, resulting from prenatal exposure to drugs of abuse more closely.
Aims of the study
The aim of this paper was to review the current magnetic resonance imaging (MRI) literature on the effects of prenatal alcohol exposure in children.
Methods
Search methods for identification of studies
An initial electronic search was conducted to identify studies. A search was conducted through the following databases: PubMed, PsycINFO and Google Scholar. Combinations of the following search terms and keywords were used to identify relevant studies: ‘alcohol’, ‘fetal alcohol spectrum disorders’, ‘fetal alcohol syndrome’, ‘FAS’, ‘FASD’, ‘MRI’, ‘DTI’, ‘MRS’, ‘neuroimaging’, ‘children’ and ‘infants’. No starting date limits were enforced to restrict the search; however, the search extended up to 15 August 2014 and papers were restricted to those that included human subjects <18 years of age as a proportion of their cohort and that were published in English language journals. Abstracts were manually examined in order to confirm relevance. Further studies were identified by searching the reference lists of the studies identified in the initial database search. This was carried out to ensure that studies that were missed in the initial search were identified. A total of 64 relevant articles were identified and included in this review.
A qualitative approach was taken for this review instead of a quantitative comparison such as meta-analysis. Reasons for this decision included the following: data required to compute effect size were not always available; the methodological detail to define ROI in different studies varied considerably, preventing clear comparisons; there were important differences in secondary variables across different studies (in particular age, gender, extent and timing of alcohol exposure and polysubstance exposure); and, finally, different analytic methods were used to report the imaging findings (e.g. voxel-based vs. ROI-based) across studies. As a result, a qualitative approach seemed more appropriate for this particular review, which covers over two decades of neuroimaging studies on the effects of prenatal alcohol exposure on the brain.
Results
Structural magnetic resonance imaging (sMRI) in prenatally alcohol-exposed children
Since the early 1990s, MRI technology has been used to report quantitative effects on the brains of children exposed to alcohol in the antenatal period. Structural neuroimaging studies of prenatal alcohol exposure have been most widely reported and 32 relevant studies were identified for this review and are listed alphabetically in Table 1.
Table 1 Structural magnetic resonance imaging (MRI) in prenatally alcohol-exposed children
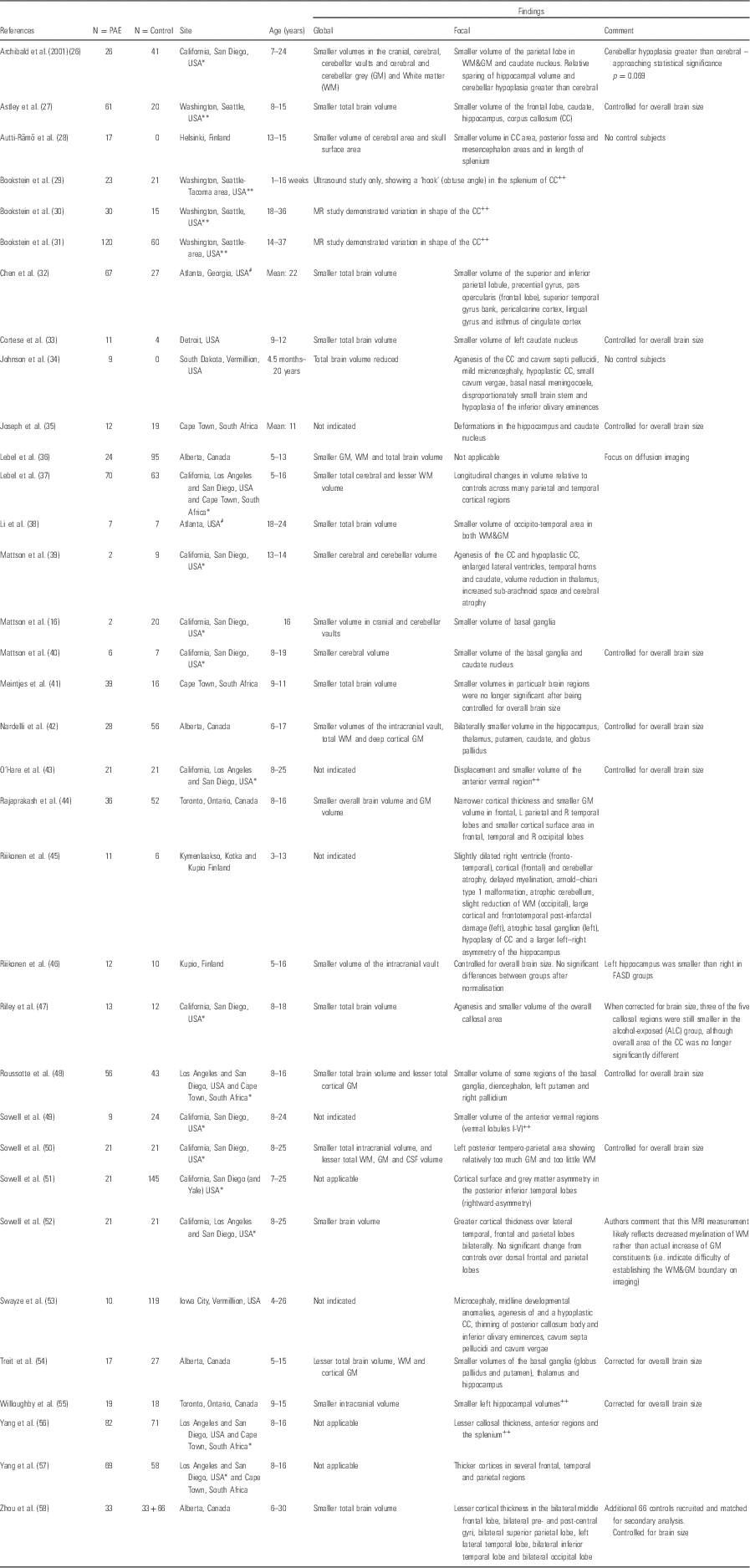
CSF, cerebrospinal fluid; FASD, foetal alcohol spectrum disorder; ROI, region of interest.
The following codes denote sample overlap from specific regions: *California/San Diego; #Atlanta; **Washington. The following codes denote report on a single ROI: ++
DTI in prenatally alcohol-exposed children
White matter in the brain provides the connections that comprise the brain’s structural neural networks. Its integrity is essential for the effective functioning of a wide spectrum of complex cognitive processes. In particular, white matter integrity has been demonstrated to play a critical role in normal executive function, attention and processing speed (Reference Wozniak, Mueller, Chang, Muetzel, Caros and Lim59–Reference Hermoye, Saint-Martin and Cosnard61).
White matter microstructure can be measured in vivo with DTI, as well as other diffusion MRI approaches, and can estimate the overall directional diffusion of water molecules along fibre pathways (Reference Pannek, Guzzetta, Colditz and Rose62,Reference Beaulieu63). Analysis of this data allows the degree of structural and organisation of areas within the brain tissue to be determined. Traditional scalar metrics derived from DTI data include FA, which quantifies the overall directionality of diffusion, and may represent variations in axon integrity and/or packing density. Mean diffusivity (MD) provides a measure of the average diffusivity and may primarily reflect myelin breakdown, decreased cellular density or increased extra- or intra-cellular volumes, although these relationships are less clearly defined in the developing brain. High FA and low MD values are typically associated with healthier neural microstructure in adults, whereas low FA and high MD values may indicate white matter pathology. However, it is also relevant to note that during brain maturation in healthy children and adolescents, axonal pruning and other biological processes may also lead to reduced FA (Reference Dubois, Dehaene-Lambertz, Kulikova, Poupon, Hüppi and Hertz-Pannier64–Reference Paus, Pesaresi and French66).
Studies reporting the effects of prenatal alcohol exposure on the white matter microstructure in children extend back 10 years. Seven studies have been reported using this modality and are detailed in Table 2.
Table 2 Diffusion tensor imaging (DTI) in prenatally alcohol-exposed children
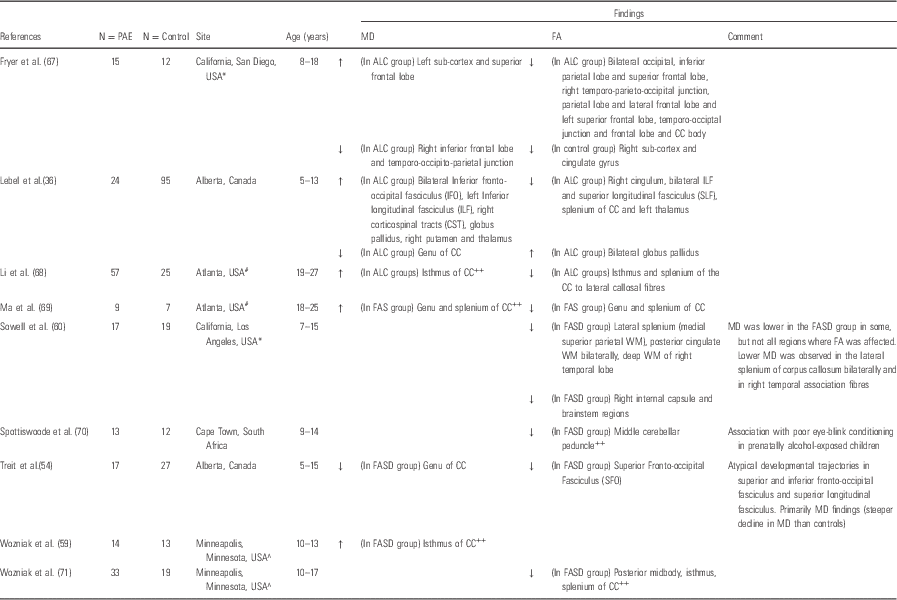
CC, corpus callosum; FA, fractional anisotrophy; FASD, foetal alcohol spectrum disorder; GM, grey matter; MD, mean diffusivity; ROI, region of interest; WM, white matter.
The following codes denote sample overlap from specific regions: *California/San Diego; #Atlanta; ^Minnesota;. The following code denotes report on a single ROI: ++.
Proton magnetic resonance spectroscopy (1H-MRS) in prenatally alcohol-exposed children
Both basic and clinical studies have begun to implicate a number of neurometabolic processes that may underlie the association between maternal alcohol abuse and subsequent negative outcomes in offspring (Reference Ikonomidou, Bittigau and Ishimaru72,Reference Roussotte, Soderberg and Sowell73). 1H-MRS is a non-invasive magnetic resonance technique that measures the concentration of several brain metabolites. Levels of individual brain metabolites may suggest abnormalities in the neuronal microstructure and/or neurometabolism. The most commonly reported metabolites include NAA, which is an indicator of neuronal integrity and or viability, choline metabolites (Cho), an indicator of neuronal-membrane turnover and myelination, creatine metabolites (Cr), energy metabolites and glutamate with its precursor glutamine (Glx), the brain’s major neuroexcitatory neurotransmitter (Reference Blüml, Wisnowski and Nelson74,Reference Ross, Colletti and Lin75). Published 1H-MRS studies include only four studies that were performed on children and adolescents, these data are presented in Table 3.
Table 3 Proton magnetic resonance spectroscopy (1H-MRS) in prenatally alcohol-exposed children
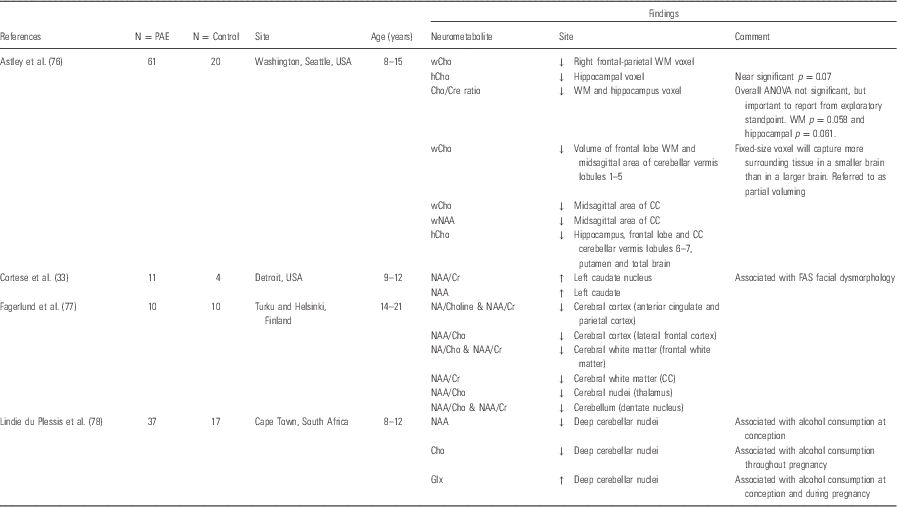
Cho, choline containing metabolites; Cr, creatine-containing metabolites; Glx, glutamate with its precursor glutamine; NAA, N-acetyl-aspartate.
Functional MRI (fMRI) in prenatally alcohol-exposed children
Increasingly, over the recent years, investigators have sought to document correlations between the functional deficits reported in children with FASD and the underlying neurobiology. Animal work (Reference Burke, Palmour, Ervin and Ptito79,Reference An and Zhang80) and human imaging studies in school-age children have demonstrated that in utero exposure to alcohol alters brain morphology and reduces white matter microstructural integrity (Reference O’Leary-Moore, Parnell and Lipinski15,Reference Archibald, Fennema-Notestine, Gamst, Riley, Mattson and Jernigan26,Reference Lebel, Rasmussen, Wyper, Walker, Andrew and Yager36,Reference Lebel, Mattson and Riley37,Reference Wozniak, Mueller, Chang, Muetzel, Caros and Lim59,Reference Fryer, Schweinsburg, Bjorkquist, Frank, Mattson and Spadoni67,Reference Sowell, Johnson and Kan81–Reference Leigland, Ford, Lerch and Kroenke85). However, there are few data on functional connectivity in these children. Functional connectivity in the imaging literature has been defined as dependencies among observed neurophysiological responses or ‘temporal correlation between spatially remote neurophysical events’ (Reference Biswal, Zerrin Yetkin, Haughton and Hyde86). In this case, intrinsic brain activity is measured in the absence of an experimental task or ‘at rest’. The ability of the brain to co-ordinate areas of activity in specific functional networks follows a developmental trajectory, reflected in the increased functional network connectivity with age in childhood and early adulthood (Reference Hagmann, Grant and Fair87). Two preliminary reports from a single cohort have described inter-hemispheric and global functional connectivity abnormalities in older children (10–17 years) with FASD.
Task-based fMRI is a direct method for investigating the function of the brain in humans. The technique measures the dynamic distribution of blood flow to specific regions of the brain during a defined motor or cognitive activity. The choice of tasks investigated has largely focussed on functional deficits previously described in children prenatally exposed to alcohol and include working memory (Reference Norman, O’Brien and Spadoni88–Reference O’Hare, Lu and Houston95), sustained attention (Reference Li, Coles, Lynch, Ma, Peltier and Hu38), response inhibition (Reference O’Brien, Norman and Fryer96,Reference Fryer, Tapert, Mattson, Paulus, Spadoni and Riley97), verbal learning (Reference Sowell, Lu and O’Hare98) and mathematical tasks (Reference Meintjes, Jacobson and Molteno99,Reference Santhanam, Coles, Li, Li, Lynch and Hu100). Rousotte and colleagues have also reported connectivity in alcohol-exposed and in polydrug-exposed children, aged between 7 and 15 years, during a working memory task (Table 4).
Table 4 Functional MRI (fMRI) in prenatally alcohol-exposed children
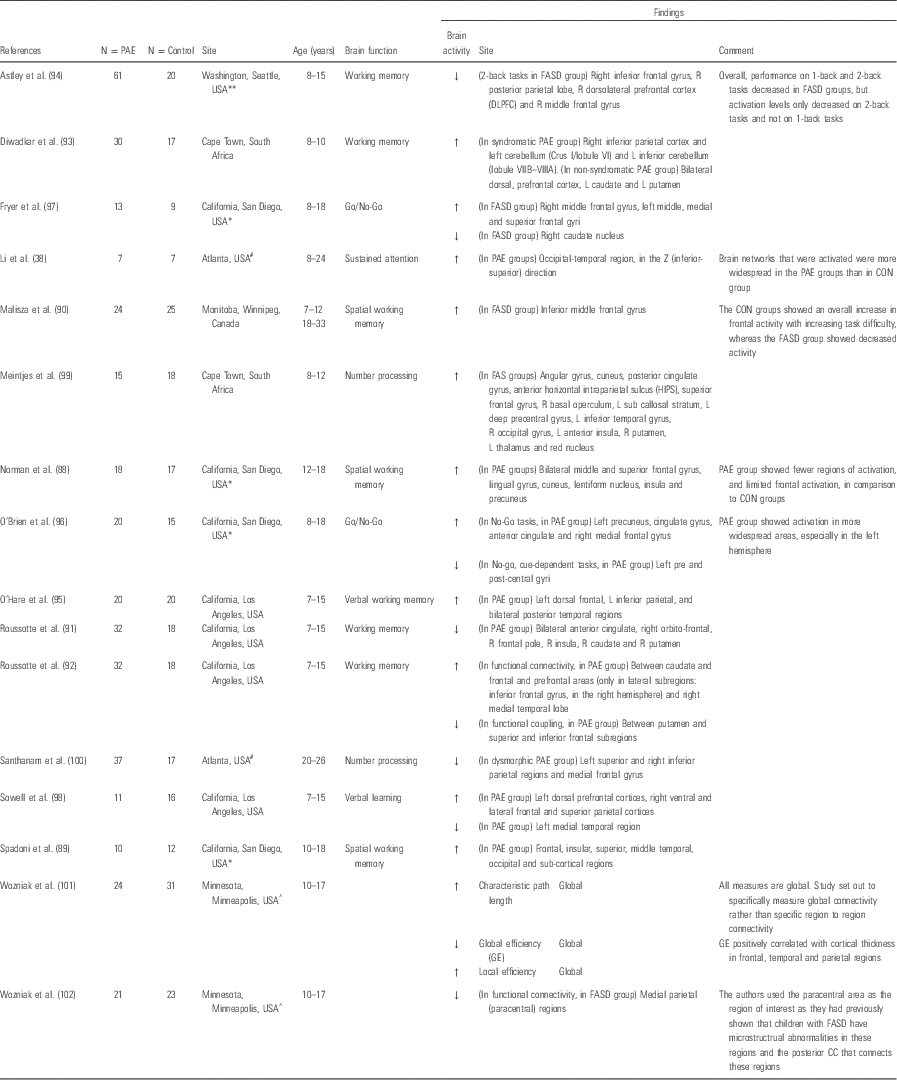
ACER, Alcohol Clin Exp Res; FASD, foetal alcohol spectrum disorders.
The following codes denote sample overlap from specific regions: *California/San Diego; #Atlanta; ^Minnesota; **Washington.
Discussion
The neuroimaging literature of the past 20 years has documented the deleterious effects of prenatal alcohol exposure on many important systems in the developing brain. These findings in children are consistent with the animal neuroimaging literature, which has demonstrated the teratogenic effects of alcohol on the immature nervous system via a number of cellular and molecular mechanisms leading to structural, functional and metabolic abnormalities in a wide spectrum of brain regions.
sMRI
Early reports on structural neuroimaging effects of prenatal alcohol exposure focussed largely on global effects and on the severe end of the FASD spectrum. Studies consistently reported smaller whole-brain volume as well as more specifically smaller volumes of both white and grey matter across the whole brain. A small minority found proportionally greater volume differences in overall deep grey matter (Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42) or white matter (Reference Archibald, Fennema-Notestine, Gamst, Riley, Mattson and Jernigan26) when controlling for overall brain volumes. In addition, although cortical thickness alterations have been reported, the direction of change remains mixed across studies (Reference Lebel, Mattson and Riley37,Reference Rajaprakash, Chakravarty, Lerch and Rovet44,Reference Roussotte, Sulik and Mattson48,Reference Treit, Lebel, Baugh, Rasmussen, Andrew and Beaulieu54,Reference Yang, Phillips and Kan56–Reference Zhou, Lebel and Lepage58). However, these findings were not specific to alcohol exposure and research focus moved on to the identification of volume and shape differences of more defined regions.
The most consistent finding across studies of structural MRI in alcohol-exposed children remains alterations in both the shape and area of the corpus callosum. Given that this structure represents the largest white matter tract in the brain, an important midline structure as well as the primary connection between the hemispheres, alterations in its shape and volume might be reasonably hypothesised in the context of alcohol exposure. Reported variations range from total absence of the structure or partial agenesis in individual cases (Reference Riikonen, Salonen, Partanen and Verho45) to more subtle between-group differences in volume (Reference Riley, Mattson, Sowell, Jernigan, Sobel and Jones47,Reference Roussotte, Sulik and Mattson48,Reference Yang, Phillips and Kan56), area or length (Reference Astley, Aylward and Olson27,Reference Autti-Rämö, Autti, Korkman, Kettunen, Salonen and Valanne28,Reference Riley, Mattson, Sowell, Jernigan, Sobel and Jones47,Reference Sowell, Mattson, Thompson, Jernigan, Riley and Toga103) and position (Reference Sowell, Mattson, Thompson, Jernigan, Riley and Toga103) relative to unexposed children. These findings are likely to represent in part the well-described vulnerability of midline structures to alcohol-induced damage in exposed individuals, but may also represent downstream effects of alterations in other areas of the brain, resulting in reduction in connections between these regions across the hemispheres. Finally, although these studies confirm that the corpus callosum is a heavily affected region during prenatal alcohol exposure, as can be seen from Table 1, the region has frequently been singled out for discrete ROI analysis, and thus effects on the brains of these exposed children may in fact be more extensive than they would seem from these reports.
Differences in grey matter volumes and/or thickness have been reported for cortical regions as well as for the deep grey matter. Frontal, parietal and temporal cortices have been implicated, although the direction of effect is not consistent between studies (Reference Lebel, Mattson and Riley37,Reference Sowell, Thompson and Peterson51,Reference Sowell, Mattson, Kan, Thompson, Riley and Toga52,Reference Yang, Roussotte and Kan57,Reference Zhou, Lebel and Lepage58). In particular, the relationships between the abnormalities of the cortical structure have been related to cognitive functional outcomes, and the differences in cortical development over time in alcohol versus control groups have been reported by Sowell and colleagues (Reference Lebel, Mattson and Riley37,Reference Sowell, Mattson, Kan, Thompson, Riley and Toga52,Reference Treit, Lebel, Baugh, Rasmussen, Andrew and Beaulieu54). This argues for the clinical importance of damage to these areas after alcohol exposure.
Other areas that have been reported as consistently affected include the deep grey matter structures. Most consistently reported was volume reduction in the hippocampi, even when corrected for total brain volume (Reference Archibald, Fennema-Notestine, Gamst, Riley, Mattson and Jernigan26,Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42,Reference Willoughby, Sheard, Nash and Rovet55). Additional studies found the hippocampi to have smaller volumes in proportion to overall brain volume reduction (Reference Astley, Aylward and Olson27,Reference Roussotte, Sulik and Mattson48). Basal ganglia volume reductions in alcohol-exposed children in comparison with their non-exposed peers have been reported by a number of authors (Reference Mattson, Riley and Jernigan16,Reference Archibald, Fennema-Notestine, Gamst, Riley, Mattson and Jernigan26,Reference Cortese, Moore, Bailey, Jacobson, Delaney-Black and Hannigan33,Reference Mattson, Riley, Sowell, Jernigan, Sobel and Jones40,Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42,Reference Riikonen, Nokelainen and Valkonen46,Reference Roussotte, Sulik and Mattson48,Reference Astley, Richards and Aylward76). Alcohol exposure effects on the caudate have been most frequently cited (Reference Mattson, Riley and Jernigan16,Reference Archibald, Fennema-Notestine, Gamst, Riley, Mattson and Jernigan26,Reference Cortese, Moore, Bailey, Jacobson, Delaney-Black and Hannigan33,Reference Mattson, Riley, Sowell, Jernigan, Sobel and Jones40,Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42,Reference Riikonen, Nokelainen and Valkonen46,Reference Roussotte, Sulik and Mattson48,Reference Astley, Richards and Aylward76), but also discrete changes in the globus pallidus (Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42,Reference Roussotte, Sulik and Mattson48,Reference Treit, Lebel, Baugh, Rasmussen, Andrew and Beaulieu54), putamen (Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42,Reference Riikonen, Nokelainen and Valkonen46,Reference Roussotte, Sulik and Mattson48,Reference Treit, Lebel, Baugh, Rasmussen, Andrew and Beaulieu54,Reference Astley, Richards and Aylward76) or the lenticular nucleus as a whole (Reference Mattson, Riley, Sowell, Jernigan, Sobel and Jones40). The implication of alcohol targeting on these sub-cortical nuclei is not well-established due, in part, to the paucity of functional outcome data linked to these changes. However, their role in critical networks regulating behaviour and impulse control among other functions could be considered a consistent hypothesis in this group of affected children (Reference Fix104).
Areas where findings have showed greater discrepancy include the thalamus, which has been reported to have smaller volumes even when correcting for brain volume by some groups (Reference Mattson, Riley and Jernigan39,Reference Nardelli, Lebel, Rasmussen, Andrew and Beaulieu42,Reference Treit, Lebel, Baugh, Rasmussen, Andrew and Beaulieu54) and changes only in proportion to overall brain volume by others (Reference Archibald, Fennema-Notestine, Gamst, Riley, Mattson and Jernigan26,Reference Roussotte, Sulik and Mattson48). The diencephalon, overall, has also been reported as displaying reduced volume (Reference Mattson, Riley, Sowell, Jernigan, Sobel and Jones40) or area (Reference Autti-Rämö, Autti, Korkman, Kettunen, Salonen and Valanne28) or no significant structural changes at all (Reference Mattson, Riley and Jernigan16).
The body of literature reporting structural brain changes associated with in utero alcohol exposure using cross-sectional study designs, described above, is now quite large. The studies have focussed on a range of ages from 5 years through to young adulthood. However, the majority of participants were either in late childhood or adolescence, and very few studies grouped participants in a narrow age-bracket. As a result, although there is reasonable consistency in the location of the PAE effects on the developing brain, there is very little clarity on when these changes have onset, the nature and functional importance of changes in specific areas at particular time points and the developmental trajectory of these changes across childhood or into later adulthood. There is established documentation of age-related changes in the developing brain, both at the structural and microstructural level (Reference Dubois, Dehaene-Lambertz, Kulikova, Poupon, Hüppi and Hertz-Pannier64,Reference Geng, Gouttard and Sharma65,Reference Gilmore, Shi and Woolson105,Reference Shaw, Kabani and Lerch106). These changes do not necessarily follow linear trajectories, and different areas of the brain appear to follow different maturational patterns (Reference Shaw, Kabani and Lerch106). The CIFASD group, in one of the only reported longitudinal studies in alcohol-exposed children, has reported altered trajectory of cortical development compared with non-exposed peers (Reference Lebel, Mattson and Riley37,Reference Treit, Lebel, Baugh, Rasmussen, Andrew and Beaulieu54). In particular, parietal and some temporal regions demonstrated either inverted or flattened volume curves over time. Of interest are the different effects that were noted at different ages in specific areas. For example, a cross-sectional comparison of these groups at 5–6 years of age may have demonstrated increased volume in the parietal cortex in prenatally alcohol-exposed children, whereas at 11–12 years of age the volume relationship may have been reversed. This study supports the view that developmental trajectories may be a better indicator of atypical brain development, and emphasises the conspicuous absence of data in children with prenatal alcohol exposure during the early years of life, which represents the most rapid period of brain growth.
DTI
DTI has proved to be a particularly useful tool in the investigation of the more subtle effects of the spectrum of alcohol and polysubstance exposure on white matter integrity of the developing brain (Reference Wozniak, Mueller, Chang, Muetzel, Caros and Lim59). The available data on DTI findings in children exposed to alcohol have consistently demonstrated reduced FA in the corpus callosum. Abnormalities in MD have also been reported, although the nature and specific location of these changes have been different across studies (Reference Sowell, Johnson and Kan60,Reference Wozniak, Muetzel and Mueller71,Reference Norman, Crocker, Mattson and Riley107). A recent review of seven DTI studies in older children and young adults exposed to alcohol prenatally reported white matter microstructural abnormalities (lower FA) in the corpus callosum, anterior–posterior fibre bundles and the cerebellum (Reference Wozniak, Mueller, Chang, Muetzel, Caros and Lim59,Reference Li, Coles, Lynch and Hu68,Reference Ma, Coles and Lynch69,Reference Wozniak, Muetzel and Mueller71,Reference Wozniak, Muetzel and Mueller71,Reference Lebel, Roussotte and Sowell82,Reference Wozniak and Muetzel108). These abnormalities have also been reported in the frontal (Reference Lebel, Rasmussen, Wyper, Walker, Andrew and Yager36,Reference Fryer, Schweinsburg, Bjorkquist, Frank, Mattson and Spadoni67), temporal lobe regions in particular (Reference Lebel, Rasmussen, Wyper, Walker, Andrew and Yager36,Reference Sowell, Johnson and Kan60), and sub-cortical structures (globus pallidus, thalamus and putamen) (Reference Lebel, Rasmussen, Wyper, Walker, Andrew and Yager36,Reference Lebel, Mattson and Riley37) (Table 2).
Very few studies have addressed associations between white matter microstructural abnormalities and specific measures of cognitive and behavioural function (Reference Sowell, Johnson and Kan60,Reference Lebel, Rasmussen, Wyper, Andrew and Beaulieu84). Alhough negative findings exist for several functional domains, Sowell et al. (Reference Sowell, Johnson and Kan81) showed associations between reduced performance on a measure of visuomotor integration and reduced FA in the splenium of the corpus callosum and parietal white matter. Lebel et al. reported significant associations between reduced FA in the left parietal lobe, cerebellum and brainstem with mathematical ability in 5- to 13-year-old children (Reference Wozniak and Muetzel108). These results suggest clinical significance of the DTI findings in these brain regions, at least in school-age children. However, existing studies have generally used relatively low angular resolution data (with between 6 and 35 gradient directions) and spatial resolution ≥2.5 mm. Thus, more sensitive non-tensor-based models have not been applied, which may have greater sensitivity. To date, almost no data exist regarding the impact of prenatal alcohol exposure in early infancy before higher-level brain networks have become established or the confounding postnatal environmental influences to which children from these backgrounds are exposed have come into play. Moreover, there are few data on when changes in white matter structural integrity have onset, regional specificity in early brain development and whether there are any early neurobehavioural associations.
1H-MRS
The first reported 1H-MRS study in children exposed to alcohol in utero reported increased NAA concentration in the caudate nucleus in a group of children with full-blown FAS. The group also reported relationships between NAA concentration and facial dysmorphometry (Reference Cortese, Moore, Bailey, Jacobson, Delaney-Black and Hannigan33). A second study in slightly older children identified decreased NAA/Cr and NAA/Cho ratios in multiple regions including the cortical and sub-cortical regions. These included parietal and frontal cortices, thalamus and cerebellar dentate nucleus as well as the frontal white matter and corpus callosum (Reference Fagerlund, Heikkinen and Autti-Rämö77). These changes in neurometabolite ratios are suggested to reflect changes in glial proliferation (Cho and Cr) rather than decreased neuronal integrity/viability (NAA) in children with FASD. A subsequent study in a larger cohort found reduced choline in the frontal and parietal white matter of children with FASD compared with exposed children without the FAS facial phenotype or cognitive/behavioural dysfunction and unexposed children. Another group has also reported reduced choline, but in the left striatal region, in a group of children with a diagnosis of FASD compared with a control group (Reference Gonçalves, de and Vasconcelos109). Finally, a well-characterised group of FASD children in South Africa were found to have lower NAA levels in the deep cerebellar nuclei associated with alcohol exposure around conception. Higher levels of alcohol consumption during pregnancy were related to reduced Cho and with increased concentrations of GLx in the deep cerebellar nuclei (Reference du Plessis, Jacobson and Jacobson78). Despite discrepencies in site and type of neurometabolites reported in these studies, these individual reports indicate alterations in the neurochemistry across important areas in the grey and white matter regions of children exposed to alcohol in utero. However, brain metabolism and associated neurochemistry are dynamic and site-specific. Certainly, age-specific changes in concentrations of these metabolites are documented (Reference Blüml, Wisnowski and Nelson74), and the findings described above represent a broad age range as well as differing approaches to clinical classification for comparison groups. In addition to the above, technical factors such as the choice of the location of voxel placement make comparison across these studies largely unhelpful.
To date, no studies have reported the use of 1H-MRS to explore the effects of prenatal alcohol exposure on the developing infant brain. This is an important period to investigate, as the neurometabolic milieu at this early stage of development is likely to have a significant impact on subsequent brain development (Reference Blüml, Wisnowski and Nelson74) and is less likely to be contaminated with environmental factors. In particular Glutamate (Glu), an excitatory neurotransmitter, also plays a key role in early life in the regulation of cell proliferation, migration and pruning (Reference Karadottir and Attwell110), and alcohol exposure has been shown to disrupt this process in animal models (Reference O’Leary-Moore, McMechan, Galloway and Hannigan23,Reference Ikonomidou, Bittigau and Ishimaru72).
Functional magnetic resonance imaging (RS-fMRI)
Functional changes in brain activity relating to specific tasks in children exposed prenatally to alcohol vary across a number of cognitive domains. Working memory is the domain that has been most widely investigated (Reference Norman, O’Brien and Spadoni88–Reference O’Hare, Lu and Houston95), although other areas of cognitive function have been described (Reference Li, Coles, Lynch, Ma, Peltier and Hu38,Reference O’Brien, Norman and Fryer96–Reference Santhanam, Coles, Li, Li, Lynch and Hu100). All these studies reported differences in the distribution of activated brain areas during a working memory task in the prenatal alcohol exposure group compared with unexposed peers even in the absence of between-group differences in the task itself. Alterations in blood flow regulation appear most consistently in the frontal regions in these studies, and, although the particular distribution of these changes are not consistent, there appears to be a more generalised pattern of activation in the prenatal alcohol-exposed children, possibly representing reduced efficiency in activation of specific neural pathways. Whether this relates to delayed functional maturation or more permanent impairment will be addressed by studies tracking these important cognitive functions over time and specifically into early adulthood.
Wozniak et al. (Reference Wozniak, Muetzel and Mueller71,Reference Wozniak, Mueller and Muetzel102), in their initial study, demonstrated that children with prenatal alcohol exposure had abnormalities in white matter microstructural connectivity in the corpus callosum compared with healthy unexposed controls, as well as a disturbance of functional connectivity in the alcohol-exposed group in this region. A subsequent analysis of the same children demonstrated further abnormalities in global measures of network connectivity using a graph theory approach (Reference Wozniak, Mueller and Bell101). The authors reported significantly higher characteristic path length and lower global efficiency in the brains of those children with prenatal alcohol exposure. These exploratory findings are an early indication that the dynamic activation of brain regions may provide key insights into the neuropathological basis of functional impairments demonstrated by children with FASD (Table 4).
The development and maturation of the central nervous system require a carefully patterned sequence of events and processes more complex and extending over a longer period than that of any other organ system. The brain is particularly vulnerable to prenatal environmental influences, which may have long-term effects on its structure and function (Reference Rodier111,Reference Rodier112). The complexity of the brain’s structural and functional networks increases rapidly in the early months of life, representing a rapid acquisition of abilities across motor, sensory and cognitive areas (Reference Pannek, Guzzetta, Colditz and Rose62,Reference Geng, Gouttard and Sharma65,Reference Hagmann, Grant and Fair87,Reference Gilmore, Shi and Woolson105). To date, little data exist regarding the impact of prenatal alcohol exposure in early infancy before higher-level brain networks have become established and before the confounding postnatal environmental influences to which children from these backgrounds are exposed have come into play. Although information is emerging on the effects of alcohol exposure on the longitudinal structural development of the brain in later childhood (Reference Lebel, Mattson and Riley37), there are few human data on when changes have onset, where they are located at this initial stage and how complex early behavioural milestones relate to functional and structural changes of the underlying neural substrate. More specifically, although preliminary studies have shown altered connectivity in the more mature brains of school-age children, the specific effects of alcohol exposure on the establishment of intrinsic connectivity in early infancy have not been explored. Characterising the connectivity of regions in the brain that are key to early neurodevelopmental functional integration, including the thalamus and the motor cortex, may, therefore, serve as a sensitive indicator of the neuropathological effects of alcohol exposure in the human infant (Reference Doria, Beckmann and Arichi113).
Conclusions
The body of evidence documenting the neuroimaging changes associated with children and young adults exposed to alcohol during the prenatal period is now substantial. Limitations common to work in this field and which are likely to have had an impact on the consistency of the results include the issue of polysubstance abuse, even though alcohol exposure was in most cases the predominant reported exposure. Although expensive, the emerging use of biological measures of alcohol exposure such as biomarkers obtained from hair or nails are likely to improve the quantification of alcohol exposure in studies going forward. The exclusion or careful control of subjects with other substance misuse may also be improved with approaches such as urine identification of excreted drugs and cotinine measurements for tobacco exposure.
Differences in age and gender between subjects in neuroimaging studies were noted (Tables 1–4). These differences are unlikely to have affected within-study results, as exposed and control groups were generally carefully matched. However, it is possible that age in particular, but also gender differences within studies, may have affected the sensitivity for detecting changes between these two groups. As has been discussed in relation to the specific imaging modalities above, a critical gap in the extant literature is the lack of neuroimaging studies in prenatally alcohol-exposed children under 5 years of age or those over 25 years of age when brain maturation has occurred across all the modalities of imaging. In addition, the dynamic nature of brain maturation and appreciation of the effects of a significant insult such as alcohol exposure on the developing trajectory of the structural and functional network argue for the prioritisation of studies that include a longitudinal approach to understanding this spectrum of effects. Hypothesis-driven studies that include longitudinal time points as well as an integrated approach using a number of modalities at one age point, neuropsychological and behavioural outcomes and links to genetic vulnerabilities are likely to provide the most robust understanding of the neurobiological effects of prenatal alcohol exposure on the developing brain. Refining our understanding at a neurobiological level is key in developing not only earlier identification of the spectrum of alcohol-exposure effects but also targeted interventions during this important window for early intervention.
Acknowledgements
K.D. selected the literature, assisted with data extraction, compiled the first draft of the manuscript and E.E. extracted data from papers. E.E., F.H., E.R., C.A., D.S., R.W. and K.L. all contributed data and to drafts of the article, with particular attention to areas of specific expertise, and all authors approved the final draft.
Conflicts of Interest
The authors report no conflicts of interest with respect to this manuscript or relevant financial disclosures.







