Significant outcomes
-
∙ Sub-chronic treatment with imipramine, venlafaxine and ketamine as monotherapy failed to evoke antidepressant-like effects in the forced swim test (FST) in Flinders sensitive line (FSL) rats exposed to time-dependent sensitisation (TDS)-stress, suggesting treatment resistance to multiple classes of antidepressants.
-
∙ Combining imipramine with either venlafaxine or ketamine produced a significant reversal of treatment resistance in all behavioural parameters in the FST.
-
∙ Only ketamine+imipramine [frontal-cortical 5-hydroxyindoleacetic acid (5HIAA) and noradrenaline (NA)], ketamine alone (frontal-cortical and hippocampal NA) and venlafaxine+imipramine (frontal-cortical NA) increased NA and 5HIAA responses versus untreated TDS-exposed FSL rats, supporting evidence of a more robust response following combination treatment.
Limitations
-
∙ Behavioural assessment of anhedonia (sucrose preference test), which has been demonstrated to be an important symptom of treatment-resistant depression (TRD), would be a valuable addition.
-
∙ This study is limited to observations made after sub-chronic antidepressant (7 days) treatment. Extending treatment duration (inadequate treatment duration is often a reason for antidepressant non-response), and increasing dosages may provide additional support for current findings.
-
∙ Applying additional biochemical measures, for example monoamine responses via in vivo micro-dialysis and/or determination of putative molecular biomarkers of TRD such as 5HT1A-receptor expression, would bolster construct validity.
Introduction
Major depression (MD) is a commonly occurring disorder with a lifetime prevalence rate of ~16% (Reference Kessler, Berglund and Demler1). Despite several classes of antidepressants being available to clinicians (Reference Philip, Carpenter, Tyrka and Price2), pharmacological management remains suboptimal. High rates of recurrence is a constant challenge, with symptom severity serving as the greatest predictor of a poor outcome (Reference Kennedy, Abbott and Paykel3). In fact, >50% of patients still experience persistent symptoms of MD after treatment with a first line antidepressant (Reference Fava4). The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that up to 30% of patients did not achieve remission despite being subjected to multiple antidepressant drug treatments (Reference Rush, Trivedi and Wisniewski5). STAR*D was designed to replicate clinical settings, and highlighted the low remission rates associated with TRD (Reference Warden, Rush, Trivedi, Fava and Wisniewski6).
Relative to MD, TRD is associated with more severe symptomatology (Reference Wijeratne and Sachdev7) as well as increased morbidity and mortality (Reference Greden8). In addition, an increased presence of somatic symptoms, for example pain and fatigue (Reference Kroenke and Price9), may predict increased treatment resistance (Reference Greden8). The impact of TRD on healthcare expenditure is proportional to the degree of resistance (Reference Russell, Hawkins and Ozminkowski10), requiring more frequent hospitalisation (Reference Crown, Finkelstein and Berndt11), increased use of pharmacotherapy (Reference Crown, Finkelstein and Berndt11) together with an increased disease burden (Reference Greden8). Despite important strides in our understanding of the neurobiology and treatment of MD, as well as increased use of antidepressants (Reference Aarts, Noordam, Hofman, Tiemeier, Stricker and Visser12–Reference Mojtabai and Olfson14), TRD remains an undeniable concern. Nevertheless, various strategies have been employed to alleviate the non- or partial response to antidepressant treatment (Reference Sharma15).
Current approaches to treating TRD include both pharmacological and non-pharmacological (e.g. electroconvulsive therapy, psychotherapy and deep brain stimulation) approaches. Drug-centred approaches are based on switching between antidepressants either in the same or across drug classes or employing augmentative drug therapies (adding a drug from a different class or with a different mechanism of action) (Reference Philip, Carpenter, Tyrka and Price2,Reference Culpepper, Muskin and Stahl16). However, it would seem that switching within or between drug classes offers limited therapeutic benefit (Reference Souery, Serretti and Calati17). Interestingly, the latter study suggests that adjunctive treatment may accelerate symptom improvement and improve remission rates, although the authors hasten to note that the success of such a strategy requires the initial drug treatment to have at least some degree of efficacy (Reference Trivedi and Daly18), and that the adjunct treatment enhances these improvements (Reference Souery, Serretti and Calati17). Potential augmentation agents include selective serotonin reuptake inhibitors (SSRIs) and selective NA reuptake inhibitors (SNRIs) (e.g. venlafaxine), atypical antipsychotics and glutamatergic drugs (e.g. ketamine) (Reference Epstein, Szpindel and Katzman19). Venlafaxine has been found to be slightly more effective than several SSRIs in patients with severe MD (Reference Bauer, Pfennig, Severus, Whybrow, Angst and Moller20,Reference Smith, Dempster, Glanville, Freemantle and Andersen21), and acts by increasing both serotonergic and noradrenergic activity (Reference Smith, Dempster, Glanville, Freemantle and Andersen21). Ketamine, on the other hand, acts as an N-methyl-D-aspartate (NMDA) receptor antagonist (Reference Naughton, Clarke, O′Leary, Cryan and Dinan22) and is associated with a proven rapid onset of action (Reference Duman, Li, Liu, Duric and Aghajanian23) and high response rate (Reference Naughton, Clarke, O′Leary, Cryan and Dinan22), with benefits demonstrated following acute and chronic treatment (Reference Naughton, Clarke, O′Leary, Cryan and Dinan22).
Psychiatric co-morbidity is a common problem in patients with MD (Reference Fava, Rush and Alpert24,Reference Ohayon, Shapiro and Kennedy25), with co-occurrence of anxiety disorders ranging between 50% and 60% (Reference Rush, Trivedi and Wisniewski5,Reference Zarate, Singh and Carlson26). Importantly, such co-occurrence is increasingly being associated with antidepressant treatment failure (Reference Fava, Rush and Alpert24,Reference Domschke, Deckert, Arolt and Baune27,Reference Wu, Chen and Yuan28). Post-traumatic stress disorder (PTSD) is one of the more commonly co-occurring anxiety disorders in MD, and is especially prevalent in TRD (Reference Rush, Trivedi and Wisniewski5). Furthermore, half of patients with PTSD have co-morbid MD (Reference Elhai, Grubaugh, Kashdan and Frueh29) with the high co-morbidity attributed to an overlap in symptoms, for example anhedonia, sleep difficulty, irritability and poor concentration (Diagnostic and Statistical Manual of Mental Disorders criteria) (Reference Elhai, De Francisco Carvalho, Miguel, Palmieri, Primi and Christopher Frueh30,Reference Gros, Price, Magruder and Frueh31), whereas both MD and PTSD are precipitated by a chronic or severe traumatic event, respectively (Reference Elhai, De Francisco Carvalho, Miguel, Palmieri, Primi and Christopher Frueh30). Such co-occurrence is also positively associated with symptom severity (Reference Gros, Price, Magruder and Frueh31) and treatment resistance (Reference Green, Krupnick and Chung32,Reference Thase and Rush33).
The complexity and heterogeneity of MD makes it unlikely that any one animal model will fully embody the behavioural and biological characteristics of the disorder. However, modifying existing models to represent specific phenotypes of the disorder may hold promise. The gene×environment hypothesis of MD has enabled the conceptualising of genetic susceptibility combined with environmental adversity as prodromal events to the subsequent development of MD (Reference Tennant34–Reference Sullivan, Neale and Kendler36). Moreover, Willner and Belzung (Reference Willner and Belzung37) emphasise that the search for treatments for TRD may require models that incorporate predisposing factors leading to heightened stress responsiveness. The co-morbidity of PTSD and MD and its association with treatment resistance is thus noteworthy. Consequently, we have recently developed an animal model of TRD by superimposing a PTSD-related paradigm, viz. time-dependent sensitization (TDS), on the Flinders Sensitive Line (FSL) rat (Reference Brand and Harvey38). FSL rats are a well-studied genetic animal model of MD (Reference Overstreet and Wegener39), whereas TDS is based on a stress re-stress procedure (Reference Oosthuizen, Wegener and Harvey40) with proven predictive, construct and face validity for PTSD (Reference Harvey, Naciti, Brand and Stein41–Reference Liberzon, Krstov and Young44). In a companion paper (Reference Brand and Harvey38), we describe how exposing FSL rats to TDS evokes more pronounced depressive-like behaviour together with altered limbic monoamine levels versus unstressed FSL rats, as well as engendering resistance to sub-chronic imipramine treatment. To extend the predictive validity of the model, we investigated sub-chronic imipramine treatment in TDS-exposed FSL rats compared with that of venlafaxine and ketamine monotherapy as well as versus imipramine plus venlafaxine or ketamine to simulate a typical TRD regime. Post-treatment cortico-limbic monoamines were analysed after behavioural analysis.
Materials and methods
Subjects
Animals were bred and housed at the Vivarium (SAVC reg. number FR15/13458; SANAS GLP compliance number G0019) of the Pre-Clinical Drug Development Platform of the North-West University (NWU). Ambient temperature was maintained at 22±2°C with a relative humidity of 40–60% and full spectrum of light in a 12 h light/dark cycle, with lights switched on at 06:00 a.m. and off at 06:00 p.m. Food and water were provided ad libitum. All experiments were approved by the AnimCare animal research ethics committee (NHREC reg. number AREC-130913-015) of the NWU. All animals were maintained and procedures performed in accordance with the code of ethics in research, training and testing of drugs in South Africa and complied with national legislation (ethics approval number: NWU-00111-12-A5).
The original colonies of FSL rats and their control Flinders resistant line (FRL) rats were obtained from Dr. David Overstreet, University of North Carolina, USA. Subjects were male adult FSL rats (n=84 for behavioural assessment and n=56 for monoamine analysis). Table 1 describes the layout of the experimental groups. Animals in all experimental groups were either subjected to the PTSD paradigm, namely TDS, or left undisturbed (unstressed) in their home cages, after which behaviour of all animals was analysed in the open field test (OFT) and FST, with subsequent monoamine analyses performed in animals naive to behavioural assessment. Animals were housed four per cage, with the TDS paradigm initiated at an age of 40 (±1) days in order to conclude the experiments while the rats were still of an appropriate weight for the behavioural assessments. Handling of the animals was initiated 1 week before starting the experimental procedure by taking bodyweight measurements daily until the last day of the study to monitor weight gain and to calculate drug dosages.
Table 1 Layout of experimental groups

FSL, Flinders sensitive line; TDS, time-dependent sensitisation; VEH, vehicle; IMI, imipramine; KET, ketamine; VEN, velafaxine.
Time dependent sensitization (TDS)
TDS is an animal model of PTSD. Animals exposed to a severely traumatic situation followed by subsequent, but less stressful, contextual reminders exhibit significant physiological and behavioural alterations that show a time-dependent sustaining or worsening in the absence of the initiating stressor (Reference Harvey, Brand, Jeeva and Stein45,Reference Yehuda and Antelman46). The TDS paradigm used in this study (see Fig. 1) incorporated an acute single prolonged stress (SPS) sequence comprising a somatosensory stressor (restraint), a psychological stressor (forced swimming with brief submersion), and a complex stress-stimuli (exposure to ether vapours) followed by re-exposure to restraint stress 7 and 14 days later (Reference Harvey, Brand, Jeeva and Stein45).

Fig. 1 Schematic outline of the treatment-resistant depression (TRD) procedure. On day 0, rats are exposed to single prolonged stress (SPS) followed by re-exposure to restraint stress on days 7 and 14. Subsequently, behavioural assessments [open field test (OFT) and forced swim test (FST)] and monoaminergic analyses [noradrenaline (NA) and 5-hydroxyindoleacetic acid (5HIAA)] are performed on day 21.
Restraint stress
Rats were placed in Perspex® restrainers (Instrument Makers, NWU) for 2 h with the tail-gates adjusted to keep each animal well-contained without impairing circulation to the limbs. The same procedure was followed on days 7 and 14 during the re-stress phase of the TDS protocol.
Forced swim stress
Rats were placed individually in cylindrical Perspex® swim tanks (Instrument Makers, NWU) containing 40 cm of ambient water (25°C) and allowed to swim for 15 min while being forcefully submerged for the last 20 s. Thereafter, animals were removed from the cylinders, dried and returned to their home cages for 15 min to recover. Forced swimming was performed 21 days before behavioural testing (only as part of the SPS procedure and not during re-stress) in the FST so that any possible conditioned response to swim stress in the FST is unlikely.
Exposure to ether vapours
After 15 min of swim stress, rats were exposed to 5 ml of 100% ether vapours in a 5 l sealed plastic container until loss of consciousness (±2 min). Ether was poured onto a paper towel at the bottom of the container with the animal placed on a raised metal platform to avoid direct contact with the substance. After loss of consciousness, the animals were immediately removed from the plastic container, returned to their home cage for observation until regaining full consciousness and then returned to their holding room. Animals were left undisturbed, thereafter only subjecting them to routine handling until re-exposure to restraint stress during the re-stress phase of the TDS protocol.
Open field test (OFT)
This test is generally performed before the FST to control for locomotor activity possibly contributing to altered swimming performance in the FST and thereby confounding interpretation of the results. The OFT was performed half an hour before subjecting animals to the FST. Rats were individually placed in a square arena (100×100×50 cm) facing the centre and their behaviour recorded for 5 min using a ceiling-mounted digital camera. The video files were subsequently analysed using EthoVision® XT software (Noldus® Information Technology, Wageningen, The Netherlands) with total distance moved applied as a measure of locomotor activity.
Forced swim test (FST)
The FST can reliably predict antidepressant-like effects after drug treatment and is considered a model of behavioural despair that is typically manifest in human MD, and expressed in rodents as a decrease in escape-driven behaviour (i.e. increased immobility) (Reference Porsolt, Anton, Blavet and Jalfre47). During behavioural analysis, rats were placed individually in cylindrical Perspex® swim tanks containing 30 cm of ambient water (25°C) for 7 min and their behaviour recorded. The first and last minute of the video files were discarded and the remaining 5 min of swimming behaviour scored for characteristic escape-directed behaviours, including swimming, climbing (struggling) and immobility. The former two swimming parameters of the FST provide useful information relating to serotonergic (swimming) and noradrenergic (climbing) directed behaviours that may inform on the mode of antidepressant action, allowing possible correlation with whole-brain monoamine levels (Reference Detke, Rickels and Lucki48).
Drug administration
After weighing all animals daily (between 09:00 a.m. and 11:00 a.m.), imipramine (Sigma-Aldrich, Kempton Park, South Africa) (Reference Wainwright, Workman and Tehrani49,Reference Wróbel, Serefko, Wlaź and Poleszak50), venlafaxine (Reference De Oliveira, Cunha and Borges51) (Adcock Ingram, Midrand, South Africa) and racemic ketamine (Reference Zhang, Liu and Qiu52) (Fresenius-Kabi, Midrand, South Africa) was dissolved in physiological saline (0.9% NaCl) and administered subcutaneously at a dose of 10 mg/kg to FSL animals exposed to TDS (see Table 1). Treatment started on day 15 (after completing the TDS protocol on day 14) and persisted for 7 days before behavioural testing commenced on the evening of day 21 (Figure 1). This duration of treatment is regarded adequate to establish an antidepressant response in rats (Reference Breuer, Groenink, Oosting, Westenberg and Olivier53–Reference Harvey, Duvenhage and Viljoen55). Stressed and unstressed control animals were injected with saline vehicle in the same manner as drug-treated animals.
Quantitative analysis of brain 5HIAA and NA
Several valid brain indices of 5-hydroxytraptamine (serotonin; 5HT) activity may be applied, including 5HT and 5HIAA levels and the 5HIAA/5HT ratio (Reference Shannon, Gunnet and Moore56). In this regard, in vivo micro-dialysis has proven to be a reliable method to directly measure extracellular levels of 5HT. However, whole and regional brain monoamine analysis provides total levels of 5HT – both extracellular and unreleased from nerve terminals (Reference Duncan57). 5HT is metabolised primarily to 5HIAA and has been demonstrated to reflect reliable insights into time-dependent alterations in 5HT response (Reference Mehlman, Westergaard and Hoos58). Moreover, 5HIAA levels have previously been correlated with 5HT function (Reference Shannon, Gunnet and Moore56), and was therefore applied as an indicator of 5HT-ergic function in the current study. Quantification of NA and 5HIAA in the hippocampus and frontal cortex of animals was performed using a high performance liquid chromatography (HPLC) system with electrochemical detection (HPLC-EC), as previously described (Reference Harvey, Brand, Jeeva and Stein45). An Agilent 1200 series HPLC (Agilent Technologies, Santa Clara, CA, USA), equipped with an isocratic pump, auto sampler and coupled to an ESA Coulochem Electrochemical detector (Dionex, Sunnyvale, CA, USA) with Chromeleon® Chromatography Management System software (version 6.8), was used. NA and 5HIAA concentrations in the tissue samples were determined by comparing the area under the peak of each marker with that of the internal standard, isoprenaline (range 5–50 ng/ml). Linear standard curves (regression coefficient >0.99) were found in this particular range. 5HIAA and NA concentrations were expressed as ng/g wet weight of tissue (mean±SEM).
Statistical analysis
Statistical analyses were performed using Graphpad Prism® 6 and IBM® SPSS® 22 software under the guidance of the Statistical Consultation Service of the North-West University. In pairwise comparisons of the behaviour (n=12 per group) and neurochemistry (n=8 per group) between treatment naive unstressed and stressed FSL animals, unpaired Student’s t-tests with Welch’s correction (normally distributed data as indicated by Shapiro–Wilk’s test for normality p>0.05) or Mann–Whitney U-tests (data not distributed normally) were performed. One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc analysis (normally distributed data) or Kruskal–Wallis ANOVA followed by Dunn’s multiple comparisons was applied to comparisons of the behaviour (n=12 per group) and neurochemistry (n=8 per group) in treatment naive and treated stressed FSL animals. Treatment was set as within-subject factor, whereas the respective behavioural and neurochemical parameters were set as between-subject factors. Significance was set at p<0.05 for all comparisons. Where Cohen’s d-effect sizes were calculated, large effect sizes are indicated by d>0.8 and very large effect sizes by d>1.3.
Results
Behaviour
In order to confirm the translational relevance of the FSL rat for MD, data and statistics relating to the behavioural comparisons made between stress and treatment naive FRL and FSL animals have been presented in the companion manuscript (Reference Brand and Harvey38), but are reproduced here for the sake of completion (see Table 2). For the remainder of this study, all data described were undertaken in FSL animals with/without concomitant exposure to TDS stress.
Table 2 Open field test (OFT), forced swim test (FST) and frontal–hippocampal monoamine data in unstressed Flinders Resistant Line (FRL) vs. Flinders sensitive line (FSL) animals
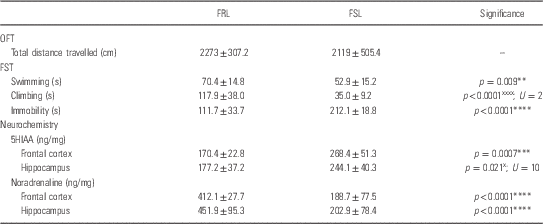
5HIAA, 5-hydroxyindoleacetic acid; TDS, time dependent sensitization; FSL, Flinders Sensitive Line.
*Unpaired Student’s t-test; xMann–Whitney U-test.
Comparison of treatment naive unstressed and TDS-exposed FSL animals is reported in Table 3, and described separately under the relevant sections below.
Table 3 Comparisons of data relating to open field and forced swim test behaviour and frontal-cortical and hippocampal markers of monoamine function in treatment naïve unstressed and time-dependent sensitisation (TDS)-exposed Flinders sensitive line (FSL) animals

5HIAA, 5-hydroxyindoleacetic acid; TDS, time dependent sensitization; FSL, Flinders Sensitive Line.
*Unpaired Student’s t-test; xMann–Whitney U-test.
OFT (Table 3, Fig. 2)
Locomotor data from the pairwise comparison between the behaviour of treatment naive unstressed and stressed FSL animals demonstrated no significant differences in overall activity (Table 3).

Fig. 2 Comparison between locomotor activity of treatment naive and treated time-dependent sensitisation (TDS)-exposed Flinders sensitive line (FSL) rats (n=12 per group). Vehicle vs. venlafaxine (d=0.94); vehicle vs. ketamine (d=1.02); vehicle vs. combinations of venlafaxine and imipramine (d=1.02) and ketamine and imipramine (d=0.9). Data are represented as mean±SEM.
Considering the various drug treatments on TDS-exposed FSL rats (Fig. 2), one-way ANOVA revealed a significant effect of treatment on the mean locomotor activity scores [F(5, 64)=2.65, p=0.03]. However, post-hoc Tukey’s analysis failed to demonstrate statistically significant differences between the means of any of the respective treatments.
FST-Swimming (Table 3, Fig. 3a)
Data from the pairwise comparison between the swimming behaviour of treatment naive unstressed and stressed FSL animals are provided in Table 3. Here we demonstrate that exposure to TDS significantly reduced the time spent swimming (p<0.0001, U=6.0).

Fig. 3 Comparison between behavioural parameters measured in the forced swim test of treatment naive and treated time-dependent sensitisation exposed Flinders sensitive line rats (n=12 per group). (a) Time spent swimming (s). Vehicle vs. venlafaxine+imipramine, ^^p<0.001; vehicle vs. ketamine+imipramine ^p<0.05; vehicle vs. imipramine, d=0.93; vehicle vs. venlafaxine, d=1.07; venlafaxine+imipramine vs. venlafaxine, d=1.08. (b) Time spent climbing (s). Vehicle vs. venlafaxine+imipramine, xp<0.05; vehicle vs. ketamine+imipramine, xx p<0.001; venlafaxine vs. venlafaxine+imipramine, xx p<0.001; ketamine vs. ketamine+imipramine, xx p<0.001; vehicle vs. imipramine, d=0.8. (c) Time spent immobile (s). Vehicle vs. venlafaxine+imipramine, ^^^^p<0.0001; vehicle vs. ketamine+imipramine, ^^^p<0.0001; venlafaxine vs. venlafaxine+imipramine, ^p<0.05; ketamine vs. ketamine+imipramine, ^p<0.05; vehicle vs. imipramine, d=1.21. xTwo-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests; ^Kruskal–Wallis ANOVA followed by Dunn’s multiple comparisons test. Data are represented as mean±SEM.
Considering the various drug treatments on TDS-exposed FSL rats (Fig. 3a), Kruskal–Wallis ANOVA revealed significant differences between the median swimming scores of animals in the respective treatment groups [H(5)=17.67, p=0.003]. As such, pairwise comparisons performed using Dunn’s procedure with a Bonferroni’s correction for multiple comparisons and adjusted p values are presented (Fig. 3a). Although a trend with a large effect size towards increased swimming behaviour was noted in animals treated with both imipramine (d=0.93) and venlafaxine (d=1.07) alone compared with vehicle-treated animals, this increase was significant in the combined venlafaxine+imipramine (p=0.005) and ketamine+imipramine (p=0.04) groups, respectively. Moreover, venlafaxine and ketamine administered as monotherapy had no effect on swimming behaviour.
FST-Climbing (Table 3, Fig. 3b)
Data from the pairwise comparison between the climbing behaviour of treatment naive unstressed and stressed FSL animals (Table 3) revealed a significant decrease in the climbing behaviour of stressed FSL animals compared with the unstressed controls (p<0.001, U=24.5).
One-way ANOVA revealed significant differences between the climbing behaviour of TDS-exposed rats in the various treatment groups [Fig. 3b, F(5, 66)=6.7, p<0.0001]. Subsequently, Tukey’s post-hoc analysis revealed significant differences in climbing behaviour between treatment naive control FSL animals and those treated with venlafaxine+imipramine (p=0.01) and ketamine+imipramine (p=0.002), respectively (Fig. 3b). Furthermore, although a trend towards increased climbing behaviour was demonstrated in animals treated with imipramine alone compared with the vehicle-treated controls (d=0.8), no such trends were demonstrated in groups treated with either venlafaxine or ketamine as monotherapies. Rather, combining both venlafaxine and ketamine with imipramine resulted in bolstered effects on climbing behaviour compared with either venlafaxine (p=0.006) and ketamine (p=0.007) administered alone, indicating an augmenting effect (Fig. 3b).
FST-Immobility (Table 3, Fig. 3c)
FSL rats exposed to TDS demonstrated a significant increase in the time spent immobile compared with unstressed FSL controls (Table 3; p<0.0001).
Kruskal–Wallis ANOVA revealed significant differences between the median immobility scores of animals in the respective treatment groups [H(5)=33.61, p<0.0001]. Subsequently, pairwise comparisons were performed using Dunn’s procedure with a Bonferroni correction for multiple comparisons of which the adjusted p values are presented (Fig. 3c). Although a trend with a large effect size (d=1.21) towards a decrease in the time spent immobile was noted in animals treated with imipramine alone compared with vehicle-treated animals, this decrease was strengthened by the concomitant administration of imipramine with either venlafaxine (p<0.0001) or ketamine (p=0.0007), respectively. Again, although neither venlafaxine or ketamine had significant effects on immobility scores when administered as monotherapy, combining both with imipramine resulted in bolstered effects on climbing behaviour compared with either venlafaxine (p=0.01) and ketamine (p=0.03) administered alone, indicating an augmenting effect.
5HIAA and NA analysis
5HIAA (Table 3, Fig. 4a)
Data from the pairwise comparisons of frontal-cortical and hippocampal 5HIAA concentrations between the treatment naive unstressed and stressed FSL animals are provided in Table 3. No significant differences were observed between the either the frontal-cortical or hippocampal 5HIAA concentrations measured. However, 5HIAA levels measured in TDS-exposed animals strongly tended towards a decrease in both the frontal cortex (d=1.07) and the hippocampus (d=0.84).

Fig. 4 Comparisons between frontal-cortical (FC) and hippocampal (H) neurochemical markers in treatment naive and treated time-dependent sensitisation exposed Flinders sensitive line rats (n=8 per group). (ai) Frontocortical 5-hydroxyindoleacetic acid (5HIAA) concentrations: vehicle vs. ketamine+imipramine, xx p<0.001; imipramine vs. ketamine+imipramine, xx p<0.001; venlafaxine+imipramine vs. ketamine+imipramine, xx p<0.001. (aii) Hippocampal 5HIAA concentrations. (bi) Frontocortical noradrenaline (NA) concentrations. Vehicle vs. venlafaxine+imipramine, xx p<0.001; vehicle vs. ketamine, p<0.0001; vehicle vs. ketamine+imipramine, p<0.0001. (bii) Hippocampal NA concentrations. vehicle vs. ketamine, ^^p<0.001; vehicle vs. imipramine, d=0.83; vehicle vs. venlafaxine+imipramine, d=0.92; vehicle vs. ketamine+imipramine, d=1.2; venlafaxine vs. venlafaxine+imipramine, d=0.82. xTwo-way analysis of variance (ANOVA) followed by Bonferroni post-hoc tests; ^Kruskal–Wallis ANOVA followed by Dunn’s multiple comparisons test. Data are represented as mean±SEM.
With respect to 5HIAA measurements in drug-treated FSL animals, one-way ANOVA revealed significant differences between the mean frontal-cortical 5HIAA concentrations measured in animals of the different treatment groups [Fig. 4ai: F(5, 41)=4.97, p=0.001]. As such, Tukey’s post-hoc analysis revealed a significant increase in frontal-cortical 5HIAA levels in ketamine+imipramine treated animals versus vehicle-treated animals (p=0.006), with none of the other treatments effective compared with the control group. In addition, frontal-cortical 5HIAA levels in rats treated with ketamine+imipramine were significantly higher than that of animals treated with imipramine alone (p=0.004), and compared with the combination of imipramine and venlafaxine (p=0.003).
Kruskal–Wallis analysis was applied in comparisons between the hippocampal 5HIAA concentrations measured in the different treatment groups (Fig. 4aii). However, no significant differences could be displayed between the median 5HIAA concentrations of any of the groups compared [H(5)=4.19, p=0.52].
NA (Table 3, Fig. 4b)
Data comparing the frontal-cortical and hippocampal NA concentrations of treatment naive unstressed and stressed FSL animals (Table 3) failed to reveal significant differences in both the frontal cortex and hippocampus. However, NA levels measured in TDS-exposed animals trended towards a decrease in the hippocampus (d=0.90).
Considering the various drug treatments on TDS-exposed FSL rats, one-way ANOVA revealed significant differences between the frontal-cortical NA concentrations in the different treatment groups [Fig. 4bi: F(5, 41)=7.6, p<0.0001]. Tukey’s post-hoc analysis showed that venlafaxine+imipramine (p=0.004), ketamine alone (p<0.0001) and ketamine+imipramine (p=0.0004) induced significantly elevated NA levels compared with vehicle-treated controls (Fig. 4bi).
Considering hippocampal NA measurements, Kruskal–Wallis analysis revealed significant differences in the median levels measured in animals across the different treatment groups [Fig. 4bii: H(5)=15.3, p=0.009]. Pairwise comparisons were performed using Dunn’s procedure with a Bonferroni correction for multiple comparisons of which the adjusted p values are presented (Fig. 4bii). Although trends towards increased NA was measured in imipramine (d=0.83), venlaxine+imipramine (d=0.92) and ketamine+imipramine (d=1.2) treated animals compared with the vehicle-treated controls, only ketamine monotherapy resulted in significantly elevated NA levels versus vehicle-treated controls (p=0.01). A large effect size was also measured in venlafaxine+imipramine versus venlafaxine alone treated animals (d=0.82).
Discussion
Several noteworthy observations have been made in this study. Exposing FSL rats to TDS exacerbates depressive-like behaviour which is depicted by reduced active coping behaviour (swimming and climbing) and increased immobility in the FST (Table 3). Where we noted a reversal of depressive-like behaviour in stress naive FSL rats with sub-chronic imipramine treatment in the companion paper (Reference Brand and Harvey38), here we found that sub-chronic treatment with imipramine, venlafaxine and ketamine as monotherapy failed to evoke a similar response in TDS-exposed FSL rats, indicating treatment resistance to multiple classes of antidepressant (Figs 3a–c). However, combining imipramine with either venlafaxine or with ketamine produced a significant reversal of treatment resistance in all behavioural parameters (Figs 3a–c). Considering monoaminergic responses, TDS-exposed FSL rats displayed a trend towards lowered 5HIAA levels in both the hippocampus and frontal cortex and lowered NA in the hippocampus (Table 3). Where we had previously noted a reversal of limbic 5HIAA and NA changes in stress naive FSL rats with sub-chronic imipramine treatment (Reference Brand and Harvey38), only ketamine+imipramine (frontal-cortical 5HIAA and NA), ketamine alone (frontal-cortical and hippocampal NA) and venlafaxine+imipramine (frontal-cortical NA) increased monoamine responses versus untreated TDS-exposed FSL rats (Figs 4ai and bi), indicating a more robust response following these combination treatments. In addition, both venlafaxine+imipramine (d=0.87) and ketamine+imipramine (d=1.12) tended to increase NA compared with vehicle-treated animals.
In the clinical setting, acute dosing with ketamine has been proven to induce rapid and robust antidepressant effects in TRD (Reference Cornwell, Salvadore and Furey59,Reference Berman, Cappiello and Anand60). More recently, however, several studies have also applied repeated dosing strategies in TRD patients which achieved superior outcomes compared with single administration approaches (Reference Aan Het Rot, Collins and Murrough61–Reference Shiroma, Johns and Kuskowski63). Likewise, in pre-clinical studies, chronic ketamine treatment has also been applied in rats using the FST compared with known antidepressants (Reference Owolabi, Akanmu and Adeyemi64) and also in animals exposed to chronic mild stress (CMS) (Reference Zhang, Liu and Qiu52,Reference Garcia, Comim and Valvassori65,Reference Parise, Alcantara and Warren66) where repeated ketamine treatment was associated with long-term anxiolytic- and antidepressant-like effects (Reference Parise, Alcantara and Warren66). Taken together, these results suggest that combining ketamine with classic antidepressants would improve antidepressant onset time with lasting and predictable effects (Reference Zhang, Liu and Qiu52). Similarly, pre-clinical (Reference Zafir, Ara and Banu67,Reference Dhir and Kulkarni68) and clinical (Reference Bauer, Pfennig, Severus, Whybrow, Angst and Moller20,Reference Smith, Dempster, Glanville, Freemantle and Andersen21,Reference Rush, Trivedi and Wisniewski69) data have demonstrated venlafaxine to be equally if not more effective than SSRIs making it a popular treatment choice in patients resistant to SSRI treatment (Reference Rush, Trivedi and Wisniewski69).
Compared with stress naive FSL rats, TDS-exposed animals presented with severely exaggerated depressive-like behaviour in the FST, characterised by significant increases in immobility and decreased coping behaviour (swimming and climbing; Table 3). TDS on its own did not adversely affect locomotor activity. In the companion paper (Reference Brand and Harvey38), we noted that sub-chronic imipramine treatment was an effective antidepressant in FSL rats. However, together with an enhanced depressive-like phenotype in TDS-exposed FSL rats, we also observed a very modest (see Cohen’s d-effect sizes) albeit insignificant behavioural response to imipramine in the FST (Figs 3a–c). Interestingly, the response to monotherapy with either venlafaxine or ketamine also proved unsuccessful. Neither of the drug treatments had a significant impact on locomotor activity, although it tended to be lower in imipramine-treated animals. Thus any observed treatment effects in the FST can be assumed to be unrelated to an indirect effect on locomotor activity. Based on these findings, and that clinically co-morbid MD and PTSD often present with TRD (Reference Green, Krupnick and Chung32,Reference Thase and Rush33), the presence of a PTSD-like paradigm in genetically predisposed animals significantly attenuates antidepressant-like response to imipramine, but also to venlafaxine and ketamine. The latter two findings with ketamine and venlafaxine are especially interesting as both agents are generally considered effective antidepressants when applied as monotherapy, and also offer improved efficacy in treatment resistance (Reference Aan Het Rot, Collins and Murrough61,Reference Thase, Gelenberg and Kornstein70). Although dose may be a reason for this observation, venlafaxine has demonstrated effectiveness in the FST after 10 days of treatment (Reference De Oliveira, Cunha and Borges51). On the other hand, it should be mentioned that sub-chronic venlafaxine treatment may be associated with non-response in the FST while still inducing monoaminergic alterations (Reference Connor, Kelliher, Shen, Harkin, Kelly and Leonard71). Previous studies with ketamine applied doses of up to 20 mg/kg twice daily for 2 weeks (Reference Parise, Alcantara and Warren66), whereas 10 mg/kg for 7 days (as applied here) have also proven to be sufficient to induce antidepressant-like effects (Reference Zhang, Liu and Qiu52). Interestingly, the latter study (Reference Zhang, Liu and Qiu52) was performed in rats exposed to a CMS protocol – a model which has been described as presenting with the attributes of TRD (Reference Jayatissa, Bisgaard, Tingström, Papp and Wiborg72). Therefore, the doses of venlafaxine and ketamine used in the current work can be regarded as effective, with ketamine demonstrating efficacy under at least some TRD-related conditions (i.e. CMS).
Exposing FSL rats to TDS stress may therefore represent a more profound state of treatment resistance that warrants a more robust treatment regimen. To test this supposition, we investigated the response to combined imipramine+ketamine or venlafaxine, considering not only superiority versus imipramine alone, but also versus ketamine and venlafaxine monotherapy. This is also a typical approach taken for a failed monotherapy treatment response in human patients (as evident in STAR*D). Where all drugs administered as monotherapy failed to induce adequate anti-immobility effects in the FST, we found that venlafaxine+imipramine and ketamine+imipramine achieved successful attenuation of depressive-like manifestations in TDS-exposed FSL rats without notable effects on locomotor activity. This conclusion is supported by a significantly reduced immobility time (Fig. 3c) as well as bolstered coping behaviour exhibited by significant increases in swimming and climbing behaviour (Figs 3a and b) following combination treatments.
The mechanism whereby the combined use of a TCA and a SNRI or an NMDA receptor antagonist may engender a bolstered response in the current model of TRD is of particular interest. Despite a plethora of up-stream signalling pathways purported to be involved in the neurobiology of MD [see (Reference Brand, Möller and Harvey73) for review], it is ultimately a resultant effect on NA and 5HT that may hold sway in the behavioural presentation of the illness and how antidepressants produce their desired effect. Considering 5HT, FSL rats present with deficits in serotonergic transmission (Reference Overstreet and Wegener39), whereas TDS in its own right adversely affects this monoamine and its behavioural sequelae (Reference Harvey, Naciti, Brand and Stein41,Reference Harvey, Naciti, Brand and Stein43), implying that TDS-exposed FSL rats may present with a profoundly compromised serotonergic system. Indeed, 5HIAA was reduced in the frontal cortex (d=1.07) and hippocampus (d=0.84) of TDS-exposed FSL rats, although narrowly missed significance (Table 3). It is interesting that clinical studies have demonstrated that relapse of MD induced by a tryptophan depleting diet occurs primarily in remitted patients taking an SSRI and not another pharmacological or behavioural treatment (Reference Moore, Landolt and Seifritz74,Reference Van Der Does75), indicating that loss of serotonergic function during treatment with serotonergic drugs mediate the relapse. As both venlafaxine and imipramine act to increase extracellular levels of 5HT (and NA) (Reference Gillman76), a synergistic action on 5HT may underlie the improved response observed in combination treatment. Drug-centred approaches for treating TRD also emphasise adding a drug with a different mechanism of action (Reference Philip, Carpenter, Tyrka and Price2,Reference Culpepper, Muskin and Stahl16). Thus, despite similar actions on NA and 5HT neuronal reuptake, imipramine has a high affinity for other neuronal receptors, such as the 5HT1A receptors (Reference Haddjeri, Blier and De Montigny77) versus the ‘cleaner’ profile of venlafaxine (Reference Muth, Haskins, Moyer, Husbands, Nielsen and Sigg78), which may explain the increased swimming behaviour observed in imipramine alone and venlafaxine+imipramine combinations versus venlafaxine alone. Also worth considering is that venlafaxine only inhibits NA reuptake at higher therapeutic doses compared with its SSRI effects across the dose range (Reference Gillman76). This may explain the absence of climbing-enhancing effects in venlafaxine alone compared with imipramine+venlafaxine, which would have provided synergistic SNRI effects.
Regarding ketamine, mechanisms involving mammalian target of rapamycin (Reference Li, Lee and Liu79) and glycogen synthase kinase-3 (Reference Beurel, Song and Jope80) may underlie its improved antidepressant response. However, ketamine is known to act via various mechanisms that may target 5HT indirectly (Reference Du Jardin, Muller, Elfving, Dale, Wegener and Sanchez81), whereas at least acute ketamine administration produces a rapid increase in the activity of locus coeruleus NA neurons through an amplification of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) transmission (Reference El Iskandrani, Oosterhof, El Mansari and Blier82). Ketamine has also been demonstrated to induce significant increases in NA release in the prefrontal cortex (Reference Kubota, Anzawa, Hirota, Yoshida, Kushikata and Matsuki83). These actions may underlie the observed additive response with imipramine in the FST. Therefore, combining imipramine with either venlafaxine or ketamine delivers an effective antidepressant response even in apparently treatment-resistant animals through broad actions on serotonergic and noradrenergic signaling. These data are important because, not only do they correlate to clinical data such as that presented in STAR*D, but reaffirms our earlier observation (Reference Brand and Harvey38) that TDS-exposed FSL rats constitute a novel and useful animal model of TRD.
Coping behaviour in the FST is thought to be mediated by the same underlying mechanisms that determine effectiveness of chronic antidepressant therapy in humans (Reference Piras, Giorgi and Corda84), highlighting that in this case both combination treatments with imipramine improved serotonergic (swimming) and noradrenergic (climbing) activities. In addition, discriminating between these coping behaviours may provide further insight into the role of monoaminergic neurotransmitter systems involved in mediating these effects (Reference Detke, Rickels and Lucki48). We have already demonstrated that FSL rats show significantly raised frontal-cortical and hippocampal 5HIAA levels as well as significantly reduced NA levels in these brain regions versus their FRL control (Table 2) (Reference Brand and Harvey38). In this study, monoamine data (Fig. 4) reveals no alterations in 5HIAA or NA in the frontal cortex following treatment with either imipramine, venlafaxine or ketamine, although ketamine increased frontal-cortical NA versus vehicle-treated animals, whereas also not markedly affecting swimming or climbing. However, ketamine+imipramine (frontal-cortical 5HIAA and NA), ketamine alone (frontal-cortical NA) and venlafaxine+imipramine (frontal-cortical NA) significantly increased 5HIAA and NA responses versus untreated TDS-exposed FSL rats (Figs 4ai and bi), indicating a more robust response following these combination treatments. Also, these combinations increased swimming and climbing (Fig. 3a,b). Considering that TCAs such as imipramine act by increasing the extracellular levels of NA and 5HT (Reference Richelson85), TDS tends to prevent these effects (Table 3) with only ketamine alone, ketamine+imipramine and venlafaxine+imipramine able to reverse the reduction in NA, whereas only ketamine+imipramine reverses TDS-associated reductions in 5HIAA (Figs 4ai and bi).
Significant increases in NA levels in the frontal cortex was measured in animals treated with venlafaxine+imipramine and ketamine+imipramine, which corresponded with increases in climbing behaviour measured in the FST. This is especially interesting considering that neither imipramine nor venlafaxine, when administered alone, were able to achieve this. However, although ketamine alone increased NA levels, this effect did not translate to climbing behaviour. Also, increased swimming behaviour was observed in rats treated with venlafaxine+imipramine, whereas neither imipramine nor venlafaxine-treated animals attained significance in this regard, despite a large effect size (d=0.93 and 1.07, respectively). Contradictions between monoamine and FST data have been reported in several animal studies in response to stress (Reference Connor, Kelly and Leonard86,Reference Walker, Burnett and Hasebe87). In fact, the paradox with respect to limbic monoamine levels and coping strategies may be indicative of adaptive changes that influence coping responses following repeated exposure to stress. However, independent of interplay between monoaminergic and behavioural responses, only augmentative treatments (venlafaxine+imipramine and ketamine+imipramine) induced significant alterations in both behavioural parameters in the FST and 5HIAA and NA responses, signifying the improved efficacy of combination versus mono-therapeutic antidepressant therapy in this model, which further lends support to its validity as an animal model of TRD.
In conclusion, combining stress sensitive FSL rats with TDS results in a treatment-resistant rat model of MD. Non-response is not only observed with the traditional antidepressant, imipramine, but also following treatment with either ketamine or venlafaxine. Exposure to TDS inhibits antidepressant response in FSL rats at both behavioural and neurochemical levels. However, combining venlafaxine or ketamine with imipramine leads to enhanced antidepressant-like effects, together with associated effects on neurochemistry. These data confirm the hypothesis that exposure of a gene-environment model of depression with a PTSD-like paradigm results in more severe depressive-like behaviour which is resistant to traditional antidepressant treatment, albeit responsive to treatment regimens which combine various mechanisms of antidepressant action. Combining FSL rats+TDS therefore holds promise for future development as a suitable animal model of TRD.
Acknowledgements
The authors wish to thank Antoinette Fick (GLP manager, Vivarium, North-West University), De Wet Wolmarans and Francois Viljoen (faculty members) for their support and guidance. Authors’ Contributions: S.J.B. performed all behavioural procedures, including treatment of the animals, performed all neurochemical analyses, undertook the statistical analysis, and prepared the first draft as well as the final version of the manuscript. B.H.H. devised the concept of the study as well as the layout of the manuscript, and finalised the pre-submission version of the manuscript.
Financial Support
The authors declare that this work has been funded by the South African Medical Research Council (MRC) (B.H.H.) and the National Research Foundation (NRF) (B.H.H.; grant number 77323). The grant holder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by NRF supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard. These funders have no other role in this study.
Conflicts of Interest
The authors declare that over the past 3 years, B.H.H. has participated in advisory boards and received honoraria from Servier®, and has received research funding from Servier® and Lundbeck®. The authors declare that, except for income from the primary employer and research funding to B.H.H. from the MRC, NRF and the above-mentioned exceptions, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional services, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.










