Significant outcomes
-
∙ Cr supplementation did not affect exploratory and locomotor behaviour in female albino mice.
-
∙ No effect of Cr supplementation on muscle functions in female albino mice.
-
∙ 3% Cr supplementation improved spatial memory in female albino mice.
Limitations
-
∙ Lack of facilities hindered us to observe changes at cellular and molecular level.
-
∙ Data from male albino mice has not been reported here.
Introduction
Creatine (N-[aminoiminomethyl]-N-methyl glycine) is a guanidine compound synthesised from glycine, arginine and S-adenosylmethionine in the kidneys, liver and pancreas and is also taken in the body through the diet in small quantities (Reference Wyss and Kaddurah-Daouk1). Cr is highly concentrated in muscular tissue (95–98%) and the remaining (2–5%) is found in other organs of the body, including brain (Reference Oliveira, Furian and Fighera2). Cr supplementation was initially confined to the athletic population, but in the last decade a significant amount of work has demonstrated the effectiveness of Cr supplementation in a variety of experimental models of neurological diseases as well as in normal central nervous system (CNS) (Reference Komura, Hobbiebrunken and Wilichowski3–Reference Allahyar, Akbar and Iqbal8).
In addition to potential role of Cr for treatment of neurological disorders, recent evidence has suggested its involvement in cerebral physiological processes, such as learning and memory. Recent studies have demonstrated that oral Cr supplementation enhances intelligence test scores, reduces mental fatigue and protects against decrease in cerebral oxygenated haemoglobin when subjects repeatedly perform a mathematical calculation (Reference Rae, Digney and Mc Ewan9–Reference Watanabe, Kato and Kato11). Long-term Cr supplementation leads to an increase in healthy life span in mice accompanied by favourable effects on neurobehavioral functioning, especially memory skills (Reference Bender, Beckers and Schneider12).
Mechanisms underlying neural function enhancement by Cr includes improved energy storage and supply. Cr seems to play a direct modulatory role in the central transmission processes (Reference Persky and Brazeau13) as in studies conducted by Almeida et al. (Reference Almeida, Salomons and Hogenboom14) have shown that Cr is released in an action-potential dependent manner, suggesting a putative role of this amino acid as a neuromodulator in the brain. Considering the important physiological role of Cr in brain metabolism, it has obvious implications for the design of therapies to maintain brain function (Reference Wyss and Kaddurah-Daouk1). Moreover, the effective participation of this compound in cognitive function is insufficiently known, so we took the lead to investigate the effect of oral Cr administration (variable doses) on exploratory behaviour and learning and memory formation in female albino mice by testing them through open field (OF) and Morris water maze (MWM) test. Muscles are among the major deposit sites of Cr in mammalian body(Reference Allahyar, Akbar and Iqbal8), and Cr supplementation can potentially affect the muscular activity, so rota rod test was also applied on the experimental animals.
Materials and methods
Subject
Female albino mice were used as experimental subjects. Breeding pairs of albino mouse were provided by Veterinary Research Institute, Ghazi road, Lahore (Pakistan). Animals were housed in animal facility at Bio Park of Bahauddin Zakariya University, Multan (Pakistan), where a breeding colony was established to generate mouse littermates used in this study. Mice were kept in cages filled with wood chips and cotton, and were maintained under controlled light and temperature conditions (14:10 h light–dark rhythm and 22±1°C) with free access to food and water. All mouse handling techniques and experimental procedure were approved by the ethical committee of Institute of Pure and Applied Biology, Bahauddin Zakariya University, Multan (Pakistan).
Experimental design
After weaning on 20th postnatal day, animals were divided into three groups; control mice were fed on normal rodent diet, group 2 was supplemented with 1% and group 3 with 3% Cr diet for 10 weeks. Cr-supplemented diets were purchased from Ssniff Spezialdäten GmbH (Sosset, Germany). Changes in body weight and diet consumed over the experimental period were also calculated. Neurological test batteries were applied at the end of diet supplementation.
Rota rod
A locally manufactured instrument with a rotating rod having a fixed speed of 40 rpm was used in the present study. Animals were individually placed on the rod up to the time they fell from the rod. Each animal was given three training and three consecutive trials with inter-trial interval of 10 min. Average time for three consecutive trials on rod were used for analysis (Reference Sunyer, Patil and Frischer15).
OF
Exploratory and locomotor behaviour was analysed during OF test by using a video monitoring system consisting of a video camcorder (XPod-058, Karachi, Pakistan) coupled to computational tracking system Anymaze (Stoelting, Illinois, USA) in an arena of 40×40 cm long with 70 cm high walls. Mice were individually placed in the middle of the chamber to be observed for 10 min. Parameters demonstrating locomotor activity and exploratory behaviour (Table 1) were recorded following Iqbal et al. (Reference Iqbal, Ali, Akbar and Iqbal16).
Table 1 Comparison of open field test results after between control, 1% and 3% Cr fed treatments after 10 weeks of special diet supplementation in female albino mice

Values are expressed as: mean±standard error of mean. N=10 for all treatments. p-Values indicate the result of one-way ANOVA calculated for each parameter. p>0.05=non-significant.
MWM
The MWM apparatus consisted of a circular pool (122 cm diameter, walls 76 cm depth) in which mice were trained to escape from water by swimming to a hidden platform (1.5 cm beneath water surface), that could only be located by using spatial memory cues. The distal extra-maze cues were attached to the room walls having different colours and dimensions and were kept constant during the whole experiment. Water temperature was maintained at 21±1°C.
The pool was divided into four quadrants (compass locations: NE, NW, SW and SE) by a computerised tracking/image analyser system (XPod-058, China) coupled to computational automated tracking system: Anymaze (Stoelting USA). The platform was placed in the middle of the NE quadrant and remained at the same position during the whole experiment, except the probe trial.
The spatial acquisition phase consisted of 16 training trials: four training trials per day and four training days with an inter-trial interval of 15 min. Mice were released randomly with their heads facing the pool wall from the four compass locations and allowed to swim and search for the platform for 120 s. If mice did not locate the platform after 120 s, animals were manually placed on the platform and allowed to remain on it for 30 s.
On the first training day, mice were given an acclimatisation training session in the water maze; mice were placed on the hidden platform, were allowed to swim for 30 s, and were guided subsequently back to the platform. The latency (time taken to reach the platform) and path length (distance mouse covers from release point to reach the platform), rest time during the trial and average speed was recorded.
One day 5, after the acquisition phase, subjects received a probe trial in which the platform was removed from the pool. Mice were released from the SW start point and were allowed to swim freely for 60 s. The path the mouse swam was tracked and analysed for the proportion of swim time and/or path length spent in each quadrant of the pool, virtual time to reach the platform, number of times crossing platform and swim speed was recorded (Reference Kipnis, Cohen and Ziv17–Reference Feldman, Shapiro and Nalbantoglu20).
Swim strategies
In order to observe any improvement in memory formation during acquisition state, seven swimming approaches were defined following Balschun et al. (Reference Balschun, Wolfer and Gass21). A mouse facing first time the water pool, tends to follow the wall of the pool with repeated wall contacts and the swim strategy of such animal is defined as wall hugging. As training proceeds, animal starts to explore the entire pool area containing the escape platform, first randomly and then selectively scans the pool central area. If animal search focusses to the target quadrant and its nearest vicinity or by directly swimming to the platform, it reflects the development of memory for the platform location. Sometimes animals start searching the pool area systematically by maintaining a constant distance from the platform location: chaining (Reference Janus22). Direct and focal approaches are categorised as spatial strategies while scanning, random and focal incorrect are categorised as non-spatial strategies. Chaining and wall hugging referred to as strategies based on recurring looping (Reference Crawley, Belknap and Collins23).
Statistical analysis
Statistical analyses were performed by utilising the statistical programme Minitab 16 (Minitab Pennsylvania, USA). Values are expressed as mean±standard error of mean (SEM) for all the studied parameters of conducted neurological tests. One-way analysis of variance (ANOVA) was calculated for the studied parameters of OF test and for MWM training as well as probe trials. The same statistical test was used to determine significant differences between treatments during rota rod test. Moreover, two-way ANOVA was applied for the parameters studied during acquisition phase of MWM considering the treatments (control, 1% and 3% Cr supplementation) as one factor and time (in days) as another factor and the trials as the repetition of the experiment. For all the significantly varying parameters during two-way ANOVA, post hoc test were calculated for training days as well as for treatments.
Results
Rota rod test
Analysis of the rota rod test results revealed that Cr supplementation did not affected the muscle function in female albino mice as the time spent on rotating rod varied non-significantly (p=0.42) when compared between the three experimental treatments (Fig. 1).

Fig. 1 Rota rod test results comparison after 10 weeks of special diet supplementation in female albino mouse. One-way ANOVA revealed no significant affect of Cr-supplemented diet on rota rod test performance (p=0.42).
OF test
Data analysis revealed that all the studied parameters to OF test varied non-significantly when compared between control, 1% and 3% Cr-supplemented experimental groups indicating that Cr supplementation did not affect the exploratory and locomotor behaviour of female albino mice (Table 1).
MWM test
Data analysis of MWM acquisition phase indicated that the distance traversed to reach the platform varied significantly between the three experimental treatments only on training day 3 (p=0.018) when 3% Cr-supplemented mice travelled maximum distance to reach the platform. The studied parameter varied non-significantly between the three treatments on training day 1 (p=0.2), day 2 (p=0.76) and day 4 (p=0.38) (Fig. 2a).
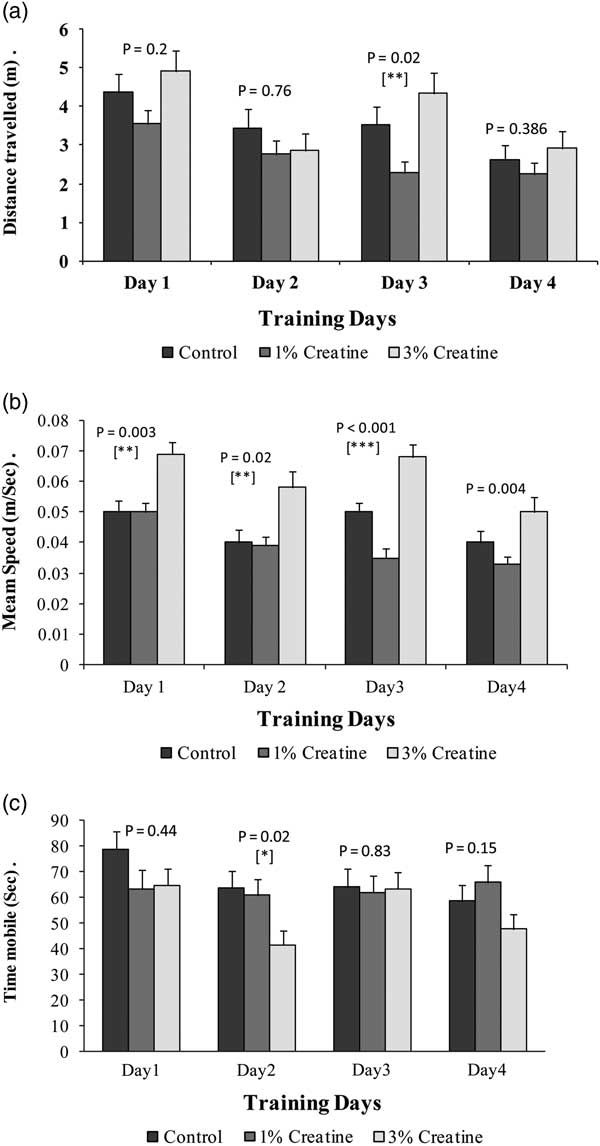
Fig. 2 Acquisition phase in Morris water maze of female albino mice, after 10 weeks of special diet supplementation: (a) distance covered, (b) mean speed, (c) time mobile. p-Values indicate one-way ANOVA results when calculated between normal, 1% and 3% Cr-supplemented female albino mice.
Significant effect of Cr-supplemented diet on mean swimming speed was observed during all four training days (day 1 p=0.003; day 2 p=0.01; day 3 p<0.001 and day 4 p=0.004) in MWM. Female albino mice supplemented with 3% Cr swam with highest average speed than other two treatments throughout the acquisition phase (Fig. 2b) indicating a better muscular activity.
Analysis of the results for the parameter time mobile during acquisition phase indicated that it varied significantly between the experimental treatments only during 2nd day of training (p=0.02) when control mice remained mobile for more time than Cr-supplemented mice. While this parameter varied non-significantly between the three treatments when compared during training day 1 (p=0. 4), 3 (p=0.8) and 4 (p=0.1) (Fig. 2c).
Data analysis for the parameter time immobile revealed that the parameter varied non-significantly between the three treatments during training day 1 (p=0.7), 2 (p=0.7) and 4 (p=0.4), whereas it significantly varied during 3rd day of acquisition phase (p=0.03) when 3% Cr-supplemented female mice remained least immobile (Data not shown here).
Two-way ANOVA was also calculated to determine the effect of training days and diet supplementation on the studied parameters. For the parameter total distance covered to reach platform, it was observed that both training days (p=0.05) and dietary supplementation (p=0.02) had significantly affected this parameter. Post hoc test analysis revealed that mice covered significantly more distance on training day 1 than remaining 3 days to reach the platform. While control group covered significantly less distance to reach platform than the Cr-supplemented treatments.
Analysis of two-way ANOVA results indicated that both the training days and dietary supplementation did not affect the mean swimming speed and time mobile parameters (p>0.05) during acquisition phase of MWM. While for the parameter time immobile during training phase, it was observed that dietary treatment has significantly affected (p=0.01) this parameter and post hoc analysis revealed that control group remained immobile for significantly less time as compared with 1% Cr treated group.
Results of probe trial revealed that female albino mice supplemented with 1% Cr transverse a greater path to reach platform, whereas mice supplemented with 3% Cr took shortest path to reach the platform but the difference observed in distance was not statistically significant. Similar results were observed for total latency as mice supplemented with 3% Cr diet had lowest latency to reach the platform (p=0.645) and also this treatment had highest number of platform entries (p=0.03) as compared with control and 1% Cr-supplemented group indicating improved learning (Fig. 3).

Fig. 3 Retention phase of female albino mice, after 10 weeks of special diet supplementation: (a) total latency; (b) number of platform entries. p-Values indicate the results of one-way ANOVA when calculated between female albino mice supplemented with normal, 1% and 3% creatine monohydrate diet.
Swimming strategies during MWM
Results of swim strategies during four training days in MWM indicated control group showed gradual improvement in spatial memory as the direct approaches to platform increased from training day 1 to 4, but random and chaining approaches were also observed indicating individual variations regarding memory formation in control mice. Mice on 3% Cr-supplemented diet showed less random strategies to approach platform than mice on 1% Cr-supplemented diet indicating a positive effect of Cr-supplemented diet. More scanning and direct approaches were observed in mice on Cr-supplemented diet towards the hidden platform with an increasing trend as the training days proceeded indicating that Cr-supplemented diet has helped in improving the spatial memory of female albino mice. The effect was more pronounced in 3% Cr-supplemented groups than the other two treatments (Fig. 4).

Fig. 4 Comparison of swim strategies used during four training days in Morris water maze to reach the hidden platform. (a) Normal rodent diet. (b) 1% creatine-supplemented diet. (c) 3% creatine-supplemented diet female mice.
Weight gain analysis
Analysis of body weight gain from weaning till end of diet supplementation experiment indicated that all the three treatments gain body weight in a similar fashion till 7th week of experiment but from this point onward, Cr-supplemented treatments gained more weight than control, but this difference in weight gain between the three treatments was not statistically significant (p>0.05) during any week of experimental duration (Fig. 5).

Fig. 5 Weight gain in female albino mice after 10 weeks of special diet supplementation following weaning at postnatal day 20.
Discussion
Recent evidences, based on experimental work and clinical trials, have suggested that Cr has important role in cerebral physiological processes, such as learning and memory. It has been reported that administration of Cr leads to an improvement in intelligence test scores (Reference Rae, Digney and Mc Ewan9) and it reduces mental fatigue when subjects were asked to repeatedly perform a simple mathematical calculation (Reference Watanabe, Kato and Kato11). As the creatine/phosphocreatine/creatine kinase system (Cr/PCr/CK) is involved in energy homoeostasis and different synaptic processes (Reference Wallimann, Wyss and Brdiczka24,Reference Schlattner, Tokarska-Schlattner and Wallimann25), it has been proposed that Cr/PCr/CK system regulates the action potentials firing speed and/or intracellular communication strength required for learning/memory formation through ATP metabolism (Reference Jost, Van and Zee26).
Rota rod is a commonly used test for rodents to determine their muscular strength and functioning. Analysis of results indicated that muscular activity of female albino mice remained unaffected when compared between mice supplemented with 1% and 3% Cr containing and those fed on normal rodent diet for 10 weeks (Fig. 1). These observations are in line with our previous findings, Allahyar et al. (Reference Allahyar, Akbar and Iqbal8), as we had reported that Cr supplementation for 10 weeks, following neonatal hypoxia ischaemia insult, did not affect the muscular function in female albino mice.
In the present study, it was observed that supplementation of 1% and 3% Cr in diet did not affect the exploratory and locomotor behaviour in female albino mice as all the studied parameters varied non-significantly between the three experimental groups (Table 1). Our findings are complementary to Allen (Reference Allen, Anci and Kanarek27) who had reported that in mice, long-term Cr supplementation did not affect locomotor and exploratory behaviour. These results are in line with those reported by Iqbal et al. (Reference Iqbal, Ali and Iqbal7) and Allahyar et al. (Reference Allahyar, Akbar and Iqbal8) who had reported that Cr supplementation did not affect the OF test performance in male and female albino mice, respectively, following neonatal hypoxia ischaemia insult.
MWM test was carried out to test the spatial memory in female albino mice. It was observed that the studied parameters (total distance, mean speed, time mobile and time immobile) showed variation between the three experimental treatments, but these variations were inconclusive as none of the three treatments had displayed a significant and/or gradual increasing or decreasing trend as compared with others throughout the training session (Fig. 2).
The results for MWM probe trial indicated that 3% Cr-supplemented mice had significantly more entries in platform areas than control and mice on 1% Cr diet (p=0.03) indicating improved spatial learning in this treatment (Fig. 3b). Importance of Cr in memory formation is evident from the findings reported by Hautman et al. (Reference Hautman, Kokenge and Udobi28) as they have mentioned that female mice heterozygous for creatine transporter deficiency showed moderate cognitive deficits. Similarly, Streijger et al. (Reference Streijger, Jost and Oerlemans29) have reported that mice lacking the UbCKmit isoform of creatine kinase reveal slower spatial learning acquisition, diminished exploration and habituation, and reduced acoustic startle reflex responses. These observations indicate that Cr synthesis, transport and metabolism have a critical role in memory formation and Cr supplementation may also influence this process.
Comparison of swimming approaches used by albino mice to reach the platform during training days of MWM revealed that random strategies of Cr treated mice were decreasing and they showed more direct and scanning approach to locate the platform as the training proceeded. A gradual decrement of random strategies and increment of direct strategies to locate the platform indicating Cr-induced learning and memory formation (Fig. 4). These observations are in agreement with those reported by Iqbal (Reference Iqbal30) as he mentioned improved learning and memory in guanidinoacetate: methyltransferase and arginine: glycine amidinotransferase knockout mice following 2% Cr supplementation.
An increase in body mass was observed in Cr-supplemented experimental treatment although it was not significantly different from control group (Fig. 5). Cr supplementation might encourage the genes and protein expression that are associated with enlargement of body parts. Thus long-term administration of Cr leads to increase in total body mass (Reference Deldicque, Atherton and Patel31,Reference Safdar, Yardley and Snow32). An increase in body weight was observed by Ferrante et al. (Reference Schlattner, Tokarska-Schlattner and Wallimann25) after oral administration of Cr in Huntington’s disease (HD) transgenic mice throughout the temporal sequence of measurements (5–13 weeks) in comparison with unsupplemented mice.
Conclusion
In summary, the current study reports that oral Cr administration, in a dose-dependent manner has partially improved the spatial memory in female albino mice, but it has no effect on rota rod and OF test performance.
Acknowledgements
These experiments were conducted as a part of PhD studies sponsored by Higher Education Commission (HEC) of Pakistan under Indigenous 5000 fellowship scheme. Authors’ Contributions: Furhan Iqbal has designed the study and revised the manuscript, Razia Allahyar has conducted the experiments and Atif Akbar has conducted the statistical analysis.
Conflicts of Interest
Authors declare that they do not have conflicts of interest of any sort.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.









