Significant outcomes
-
∙ Internet Addiction Inventory (IAT) affected the response to more rewarding cues, with increased performance [reduced response times (RTs)] in Go/No-Go task.
-
∙ High-IAT showed an increasing left frontal activity compared the Low-IAT in response to rewarding stimuli in Go/No-Go task.
-
∙ Behavioural Activation System measure were related to IAT and they were predictive of RTs and alpha modulation as a function of high/low-IAT.
Limitations
-
∙ The sample of this study is sub-clinical, it did not properly considered pathological internet abusers, thus it was explored the attitude of addictive behaviour instead of pathological behaviour.
-
∙ The spatial resolution with electroencephalographic is lower than neuroimaging techniques like fMRI.
-
∙ The ecological validity of Go/No-Go task could be lower than other stimulation conditions, which might allow to compare different types of stimuli related to the gambling effect.
Introduction
Internet addiction (IA) has been considered a specific impairment that involves online and/or offline computer misuse (Reference Zhou, Lin, Du, Zhao, Xu and Lei1,Reference Han, Lyoo and Renshaw2). It was suggested that IA should be classified as one category of behavioural addiction considering its clinical profile, comorbidity, and neurobiological aspects (Reference Grant, Marc, Weinstein and Gorelick3). A main factor that seems to be implicated in addiction was the rewarding effect and ‘reward bias’ of potential rewarding cues, such as substance, but also videogames or gambling task condition in the case of IA (Reference Park and Lee4,Reference Yen, Cheng-Fang, Chen, Chang, Yeh and Ko5). Indeed, it was suggested that some personality traits, such as reward sensitivity and impulsiveness, may have an important role to play in explaining the drug abuse and fallacy in decision making (Reference Caseras, Avila and Torrubia6). In addition (Reference Chen, Huang and Yen7) brain-imaging studies of addictive behaviours have identified a key involvement of the prefrontal cortex (PFC) through its regulation of the limbic reward regions as well as its involvement in a higher-order executive function (Reference Knyazev8). Thus addictive behaviour and IA may be explained by more receptiveness to the reinforcing effect of rewarding stimuli. Indeed, high reward sensitivity was shown to contribute to drug abuse vulnerability (Reference Balconi and Finocchiaro9–Reference Dawe and Loxton13).
On the one hand, the reward deficit syndrome was proposed as a possible contributing factor to the development of substance abuse disorders (Reference Dawe and Loxton13). Drug dependence may be related to greater receptiveness to the reinforcing effect of drugs and other rewarding stimuli. On the other hand, reward motivation significantly and directly correlates with drug addiction (Reference Balconi, Finocchiaro and Canavesio14,Reference Knyazev8). At this regard, a strong relationship was also shown between impulsivity, drug-dependence and Behavioural Activation System (BAS) and Behavioural Inhibition System (BIS) (Reference Dawe and Loxton13). In fact, BIS and BAS measures represent a promising construct to test subjective reward-sensitivity based on neurophysiological correlates (Reference Balconi, Falbo and Conte15–Reference Gray and McNaughton25). Previous findings provide support for the role of Gray’s BAS in mediating approach behaviour and dependence as associated with the drive to consume rewarding substances or to be exposed to rewarding conditions (Reference Blum, Braverman and Holder26,Reference Dawe and Loxton13,Reference Smillie, Loxton and Avery27).
Specifically, three underlying types of neurophysiological deficits have been identified in the case of reward vulnerability: hyperactivity in the emotional system, mediated by frontal and medial structures, such as the orbitofrontal cortex, anterior cingulate cortex and amygdala, which exaggerate the rewarding impact of external reinforcing cues; anomalous brain activity in the prefrontal cortex [and mainly the dorsolateral prefrontal cortex (DLPFC)], which predicts the long-term consequences of a given action (Reference Balconi and Finocchiaro9,Reference Bechara and Martin28) a specific dysfunction in the dopaminergic mesolimbic reward system which can elicit conditioned attention allocation for dependence-associated stimuli rendering them especially salient (Reference Adinoff29). Indeed, deficient mesolimbic reward system and prefrontal cortex activation is reported in substance abusers and impulsive individuals (Reference Limbrick-Oldfield, van Holst and Clark30,Reference Scheres, Milham, Knutson and Castellanos31). Direct association was also found between the BAS subscales (BAS Drive, Fun Seeking and Reward Responsiveness) and IA (Reference Yen, Ko, Yen, Chen and Chen32,Reference Yen, Cheng-Fang, Chen, Chang, Yeh and Ko5). A close relationship was also shown between impulsivity and the BAS construct in substance abuse [for a review, (Reference Dawe and Loxton13)].
The cortical correlate of BIS/BAS system is the PFC, and, whereas the left PFC was shown to be more implicated in approach-related motivations and rewarding conditions, the right PFC was found to be more involved in withdrawal-related motivations and inhibitory mechanisms (Reference Balconi and Mazza19,Reference Balconi and Mazza21,Reference Davidson33,Reference Harmon-Jones34). Both approach and withdrawal motivations are paralleled by the reward and punishment contingencies. Indeed, also frontal electroencephalographic (EEG) asymmetry has been hypothesised to relate to appetitive (approach-related) and aversive (withdrawal-related) motivation and emotion, with heightened approach tendencies reflected in left frontal activity and heightened withdrawal tendencies reflected in relative right frontal activity (Reference Davidson35–Reference Wheeler, Davidson and Tomarker38). However, no previous study has deeply considered the significance of rewarding mechanisms in the case of IA in relationship with a possibile lateralisation effect and BIS/BAS measures (Reference Balconi, Finocchiaro, Canavesio and Messina39). Therefore, we tried to relate this motivational system to the hemispheric lateralisation effect, that is the contribution by left (more reward-related) versus right (more avoidance-related) hemisphere to the motivational components which support IA behaviour. Due to the controlateral inhibition between the hemispheres, the lateralised approach and withdrawal or punishment-reward system are mutually inhibitory. Thus activation of one system will result in the inhibition of the former. Previous research found that patients after disruption of the right, lateral PFC, choosing a larger potential reward even at a greater risk of penalty (Reference Knoch, Pascual-Leone, Meyer, Treyer and Fehr40). Resting EEG studies have shown that frontal hemispheric activation asymmetry in favour of the right PFC reflects an individual predisposition to respond in terms withdrawal-related behaviour (Reference Davidson33,Reference Harmon-Jones34).
Alpha power modulation may be considered a valid measure of brain activation, and it was largely applied to find distinct responsiveness by the two hemispheres to different cognitive or emotional tasks (Reference Balconi and Mazza21). About the frontal system, reduction in alpha power (that is more cortical activation) in the left frontal brain was found after reward trials, whereas punishment conditions induced reduction in alpha power in the right frontal brain (Reference Buss, Malmstadt, Dolski, Kalin, Goldsmith and Davidson41–Reference Sobotka, Davidson and Senulis43). To test this lateralised effect based on IA and BAS construct a specific attentional inhibitory task was adopted, that is the Go/NoGo task, that can be defined as the act of withholding or terminating a behavioural response and it is considered to be governed by a cognitive inhibitory process (Reference Logan, Cowan and Davis44).
However whether and how web addiction is related to rewarding mechanisms in response to Go/NoGo is actually unexplored and unexplained (Reference Kamarajan, Rangaswamy and Cholrian45). Specific and predictive measures as Internet Addiction Inventory (IAT) (Reference Young46) as a vulnerability marker of potential AI were applied to characterise a sample of young subjects presenting high- or low-IA profile, during the performance of a Go/NoGo task in response to specific potentially rewarding cues (videos representing online gambling and videogames or neutral contexts as sport game). Indeed, IAT measures the subjective profile in term of absence or presence of IA, furnishing specific cut-off (from absent to severe IA). Whereas the low-IAT shows no IA, high-IAT may reveal addiction vulnerability from moderate to severe (Reference Young46).
Alpha frequency band (8–10 Hz) and brain activation in specific cortical sites and personality trait (BIS/BAS) were considered as predictive components to explain a potential web addiction profile. First a dynamic modulation of alpha band was expected related to the BAS construct and in response to more rewarding conditions. We supposed, that in association with more rewarding stimuli, high-BAS might show an increased alpha reduction (higher activity) within the left hemisphere in comparison with low-BAS subjects. Thus, their increased responsiveness to more ‘rewarding’ choices may be supported by the unbalance between the left and the right hemisphere, in favour to the left one.
Second, as revealed by previous analysis on different forms of addiction, it was supposed that the inhibitory control deficits and rewarding bias in response to rewarding cues should be reported in the case of increased AI profile (higher AI questionnaire scores) and high-BAS, mainly in response to GO and rewarding cues (with decreased alpha). In contrast, NoGo trials should show an increased difficulty in control and impulse inhibition in high IA and high-BAS. Indeed whereas in control subjects prefrontal decreased activity is generally expected to support the control functions (lower prefrontal responsiveness to inhibit response behaviour, with increased alpha) indistinctly for each stimulus, in IA and high-BAS we should have a reduction of this inhibitory behaviour (increased cortical response, higher alpha, similarly to Go condition), mainly for more rewarding cues and more specifically localised within the left PFC.
Aims of the study
The present research aimed to explore the contribution of rewarding bias (by Go/No-Go task) in IA by distinguishing higher IAT and lower IAT, and secondly to verify whether the motivational system (BIS-BAS) could be related to the hemispheric lateralisation effect, that is the contribution by left (more reward-related) (vs.) right (more avoidance-related) hemisphere to the motivational components which support IA behaviour and the relationship with a possible lateralisation effect. Finally, we aimed to verify that BAS measure would predict the performance in Go/No-Go task and would be related to IAT.
Material and methods
Participants
Twenty-eight volunteers took part in the study (age range=19–25, M=24.77, SD=0.99, 13 women). All subjects were undergraduate students at the Catholic University of Milan and were right-handed, with normal or corrected-to-normal visual acuity. Exclusion criteria were history of psychopathology (Beck Depression Inventory, BDI-II 1996) (Reference Beck, Brown and Steer47) for the subjects or immediate family members. In a preliminary phase of the research, two expert clinicians applied a semi-structured interview and evaluated the general psychopathological profiles of the subjects and their direct family’s members. The interview included questions on suicidal ideation, psychosis; it was also considered the absence of documented co-morbid personality disorders or mood disturb. No specific neurological or psychiatric pathologies were observed by clinical colloquium. Other addictive behaviours were excluded from the sample. A specific questionnaire was submitted to explore the drug and internet use by the subjects. They were interviewed to collect information about their behaviour about drug. The Interview was also used to examine the presence and severity of drug use. The interview evaluates three parameters: the intensity, frequency, and duration of the use of a series of substances, including alcohol, amphetamines, cannabis, cocaine, heroin, MDMA, and methadone.
About internet, their usual behaviour was considered. They were generally high-user and with a good expertise on web system. None was an internet non-user. However, they showed different profiles, as reported by IAT questionnaire which includes specific details on internet use/abuse (quality of use, time of use, etc.). All participants gave informed written consent for participating in the study, and the research was approved by the Ethical Committee (Department of Psychology) of the institution where the work was carried out.
Procedure
The participants sat on a comfortable chair in front of a Pc screen (1280/1024 pixel). The Pc was placed ~80 cm from the subject, with a visual horizontal angle of 4° and a vertical angle of 6°. They were instructed to the Go/No-Go task, prior to record EEG data. They were informed that the task consisted in four sessions and that, at the beginning of each session, would appear a black screen with instructions indicating which letter (M or W) represented the Go (press the button) and which the No-Go (do not press the button) condition. In order to familiarise with the task, the participants completed a short session of 20 trials (70% Go and 30% NoGo) on a black background. After the Go/No-Go task, the participants were submitted to a debriefing phase, with the post-evaluation questionnaires (IAT; BIS/BAS; STAI-Y; BDI-II).
Stimuli
In the experimental task the stimuli were two capital white letters (M and W; size of 500×400 mm) in Times New Roman font and background pictures (gambling-related, videogames-related, and neutral contexts) (Fig. 1) displayed on a 15-inch monitor. During a pre-testing phase, 40 pictures were selected from internet, and they were balanced for dimension, brightness and net colour with adobe Photoshop 8.0. After that, 30 voluntaries, matched with age and sex with the experimental group, evaluated these pictures for gambling- and videogames-related context, considering four dimensions: relevance, familiarity, valence and arousing power. Participants were asked to rate on a scale of five points (from zero ‘not at all’ to five ‘extremely’) the following questions for each picture: (1) How much the picture could be related to gambling/videogames? (2) How much time do you usually spend in the activity represented by the picture? (3) Could you indicate the degree of pleasantness/unpleasantness of the picture? (4) Could you indicate the degree of emotional involvement that you feel because of the picture? Finally, 18 pictures were selected and categorised into four types: six pictures (e.g. various sport scenes not related to gambling or videogames) with low scores on relatedness with gambling and videogames and emotional valence/arousal were selected for neutral condition; six pictures with high scores on relatedness, valence (positive) and arousal (high) were selected for gambling-related condition (e.g. simulating interfaces gambling sites online) and six pictures for video games-related condition (e.g. the most famous and recent video games online).
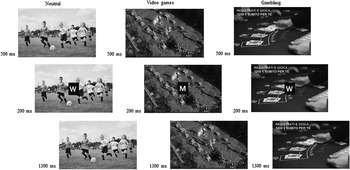
Fig. 1 Go/No-Go task. Each trial consisted of the presentation of a background picture (neutral, gambling and videogames) for 500 ms, then the letter M or W appeared in the centre of this picture for 200 ms, successively the background screen remained for 1300 ms before to start another trial with a different background picture.
Go/No-Go task
The Go/No-Go task was a modified version of the experimental task used by Petit et al. (Reference Beck, Brown and Steer47) and it was composed of four blocks of 120 stimuli per each, which were divided in 84 Go trials and 36 No-Go trials for each session. The blocks consisted of randomised presentation of background pictures from three different contexts: gambling (G), videogames (VG), and neutral (N) for 500 ms. Successively the letter M or W appeared in the centre of this background picture for 200 ms, and then the initial background picture came back for 1300 ms (Fig. 1). Therefore, participant had a maximum of 1500 ms to press the button before the next letter appears. The letters were presented in a random order to ensure the same amount as a percentage of the trials Go (70%) and No-Go (30%) for each block and category. In order to familiarise with the task, before the first session, a black screen with instructions reported which letter (M or W) represented the Go (M, press the button) and which the No-Go (W, do not press the button). Participants were required to press a button as fast as possible when they saw the Go stimulus appearing at the centre of the screen and to withhold the response for the No-Go stimulus. Moreover they were asked to reduce moving and blinking during the task in order to diminish the noise during the EEG registration. Each participant completed a total of 480 trials.
Data analysis
BIS/BAS scores
BIS and BAS scores were calculated for each subject (Reference Petit, Kornreich, Noël, Verbanck and Campanella48). The evaluation included 24 items (20 score items and four fillers, each measured on four-point Likert scale), and two total scores for BIS (range=7–28; seven items) and BAS (range=13–52; 13 items). BAS also includes three subscales (Reward, five items, Drive, four items, and Fun Seeking, four items). Based on these measures, two total scores (BIS and BAS total) and three BAS subscale scores were calculated. The mean and standard deviation values for each scale were, respectively: for BIS: 16.54 (0.87); BAS: 37.12 (1.12); Reward: 14.28 (1.20); Drive: 14.01 (1.11); Fun Seeking: 14.98 (1.16). Finally, Cronbach’s α was calculated for BIS (0.89) and BAS (0.90) and separately for each BAS subscale (Reward 0.86; Drive 0.81, and Fun Seeking 0.84).
IAT scores
Internet Addiction Test (IAT) (Reference Young46) was created according to the diagnostic criteria of the DSM-IV for pathological gambling and it was adapted for the diagnosis of IA. The questionnaire consists of 20 items measured with four-points Likert scale, ranging from ‘never’ (0) to ‘always’ (5). Once the subject has answered all the questions, the numbers of response were summed. The score is valued according to the cut-off: score between 0 and 30 (none): internet usage below the average; score between 31 and 49 (mild): an average internet user, which can sometimes happen to surf the net a bit too long but without losing control of the situation; score between 50 and 79 (moderate): the person already has several problems because of the internet and it should reflect on the impact these issues have on his life; score between 80 and 100 (severe): the use of the internet is excessive and is causing considerable problems to the person. The Cronbach’s α coefficient is from 0.75 to 0.92 (Reference Leone, Pierro and Mannetti49,Reference Laconi, Rodgers and Chabrol50). Subjects self-administrated the questionnaire after completion of the experimental task. Two sub-groups of subjects were created based on this total score: high-IAT with score more than 50 (N=12, M=53.26; SD=9.88; range=55–90); low-IAT with score <50 (N=16, M=36.58; SD=8.54; range=20–48). Gender was balanced across-group.
EEG recordings and data reduction
During task execution, EEG recordings were performed with a 64-channel DC amplifier (SYNAMPS system) and acquisition software (NEUROSCAN 4.2). An ElectroCap with Ag/AgCl electrodes was used to record EEG from active scalp sites referred to the earlobes (10/20 system of electrode placement) (Reference Pfurtscheller51,Reference Jasper52). Data were acquired using a sampling rate of 500 Hz, with a frequency band of 0.01–50 Hz. An off-line common average reference was successively computed to limit the problems associated with the signal-to-noise ratio (Reference Pascual-Marqui53,Reference Ludwing, Miriani, Langhals, Joseph and David54). Additionally, two EOG electrodes were sited on the outer canthi to detect eye movements. The impedance of the recording electrodes was monitored for each subject prior to data collection and was always below 5 kΩ. After performing EOG correction and visual inspection, only artefact-free trials were considered (rejected epochs, 3%). The signal was visually scored, and portion of the data that contained artefacts were removed to increase specificity. Blinks were also visually monitored. Ocular artefacts (eye movements and blinks) were corrected using an eye-movement correction algorithm that employs a regression analysis in combination with artefact averaging (Reference Pascual-Marqui, Michel and Lehmann55,Reference Semlitsch, Anderer, Schuster and Presslich56). The digital EEG data (from all 64 active channels) were bandpass filtered in the alpha frequency band (8–12 Hz) (band-pass filtering 96 dB/octave rolloff, warm-up filter left and right to 100 ms). To obtain a signal proportional to the power of the EEG frequency band, the filtered signal samples were squared (Reference Pfurtscheller51,Reference Crean, De Wit and Richards57). An average absolute power value for each experimental condition was calculated, using the time window of 0–500 ms. A fast Fourier transform method (Hamming window: length 10%) was used to obtain estimates of spectral power (μV2) in the 1 Hz frequency bins for each electrode site. Spectral power values were averaged across all epochs within a single baseline and were then transformed to power density values. All power density values were log transformed to normalise the distribution of the data after the subtraction.
sLORETA
To localise the source of neural activity, we used the low resolution electromagnetic tomography (sLORETA) method (Reference Pascual-Marqui53,Reference Pascual-Marqui, Michel and Lehmann55,Reference Gable, Reis and Elliot58). It solves the inverse problem based on the assumption that the smoothest possible activity distribution is the most plausible one. Specifically, an improved version of standardised weighted sLORETA was applied (swLORETA) (Reference Crean, De Wit and Richards57,Reference Harper, Malone and Bernat59). This method computes the current density (A/m2) according to the digitised probability atlas as the linear, weighted sum of the scalp electric potentials, and it assumes neither a limited number of dipolar point sources nor distribution on a known surface. Topographical voltage maps of bands were made by plotting colour-coded isopotentials obtained by interpolating voltage values between scalp electrodes at specific time interval (0–500 ms.). The source space used five-point grid spacing (the distance between two calculation points), and the estimated signal to noise ratio (which defines the regularisation) was 3. In the present research, we calculated source localisation for every subject and condition. Voxel-wise nonparametric statistics were used. Direct comparisons were successively conducted between the Go/NoGo condition.
Results
The statistical analyses were subdivided in four steps: a first set of correlational analysis finalised to explore the relationship between to BAS (and BAS-subscales) and IAT measure. A second set of analysis of variance (ANOVA), applied respectively to the dependent measures of RTs and alpha frequency band, in response to Go/NoGo task and to different stimulus condition. A third set of analysis finalised to explore the cortical localisation of alpha band (sLORETA). Finally a set of stepwise multiple regressions was applied to BAS measure as predictors of RTs and alpha modulation.
Correlational analysis BAS-IAT
Pearson’s correlation analysis (across-subject correlations) was applied to BAS and IAT measures. There was a significant positive correlation between BAS and IAT (r=0.602; p<0.001). In addition, BAS-Reward subscale was highly correlated with IAT (r=0.596; p<0.001). BAS scores were always correlated with the three BAS-subscales (p<0.001). No other Pearson value was statistically significant.
ANOVAs
Since from a preliminary analysis no significant differences were found between the experimental conditions based on number of errors (incorrect responses; omissions and commissions), we considered only the RTs as behavioural measures.
RTs
The behavioural measures of RTs were subjected to a three-way repeated measures ANOVA, in which the between-subject IAT (Reference Han, Lyoo and Renshaw2) and the within-subjects factors Go/NoGo (Reference Han, Lyoo and Renshaw2), and stimuli (Reference Grant, Marc, Weinstein and Gorelick3), were applied to the RTs. Errors associated with inhomogeneity of variance were controlled by decreasing the degrees of freedom using the Greenhouse–Geiser epsilon. Post hoc analysis (contrast analysis for ANOVA, with Bonferroni corrections for multiple comparisons) was applied in case of significant effects.
Significant effects were found for Go/NoGo [F(1,27)=11.67, p=0.001, η2=0.40], stimuli [F(2,27)=11.87, p=0.001, η2=0.40], and IAT×Go/NoGo×stimuli [F(2,54)=13.29, p=0.001, η2=0.42] (Fig. 2). About the main effects, Go condition revealed reduced RTs compared with NoGo. Moreover, as revealed by post hoc analysis, reduced RTs were found for gambling and videogames [respectively F(2,27)=9.33, p=0.001, η2=0.37] than neutral stimuli. About the significant interaction effect, simple effects revealed reduced RTs for videogames [F(1,27)=9.10, p=0.001, η2=0.35] and gambling stimuli [F(1,27)=8.60, p=0.001, η2=0.34] in Go for high-IAT more than low-IAT. Similarly reduced RTs were found for videogames [F(1,27)=9.09, p=0.001, η2=0.35] and gambling stimuli [F(1,27)=8.70, p=0.001, η2=0.33] in NoGo condition for high-IAT more than low-IAT. In contrast, in NoGo condition low-IAT showed increased RTs than in Go condition in response to gambling stimuli than neutral ones [F(1,27)=6.98, p=0.001, η2=0.30]. No other main or interaction effect was statistically significant.

Fig 2 Response times (RTs) mean values as a function of Internet Addiction Inventory (IAT) category, stimulus type and Go/NoGo task.
Alpha frequency band analysis
Alpha band measure was subjected to a five-way ANOVA, in which the between-subjects IAT (Reference Han, Lyoo and Renshaw2) and the within-subjects Go/NoGo (Reference Han, Lyoo and Renshaw2), stimuli (Reference Grant, Marc, Weinstein and Gorelick3), localisation (Reference Park and Lee4), and lateralisation (Reference Grant, Marc, Weinstein and Gorelick3) factors were applied to the dependent variable of band power. Localisation (four sites: frontal, central, temporo-parietal, and occipital) and lateralisation (three sides: left, central, and right) factors were created. Specifically, we measured left, central and right frontal (F3, Fz, F4), middle-central (Cz, C3, C4), temporo-parietal (P3/T7, Pz, P4/T8; the left and right localisations were obtained as the mean value of parietal and temporal sites) and occipital (Oz, O1, O2) brain activity.
Significant IAT [F(1,27)=7.85, p = 0.001, η2=0.34], Go/NoGo [F(1,27)=8.11, p=0.001, η2=0.36], and stimuli [F(1,27)=9.32, p=0.001, η2=0.37] main effects were found (Figs 3a and b). Indeed there was a significantly decreased alpha for high-IAT compared with low-IAT, for NoGo compared with Go condition and in response to gambling [F(1,27)=8.16, p=0.001, η2=0.36] and videogames [F(1,27)=7.13, p=0.001, η2=0.33] stimuli than neutral ones. In addition also interaction effects IAT×stimuli [F(1,54)=7.11, p=0.001, η2=0.32], IAT×Go/NoGo [F(1,27)=9.03, p=0.001, η2=0.38], and IAT×Go/NoGo×stimuli×localisation×lateralisation [F(1,108)=7.16, p=0.001, η2=0.32] were significant at the analysis. The post hoc comparisons showed the significant decreased alpha (increased brain activity) for high-IAT in Go for rewarding cues [respectively videogames (F(1,27)=7.98, p=0.001, η2=0.34, and gambler (F(1,27)=8.08, p=0.001, η2=0.36) compared with neutral stimuli] within the frontal left sides. In contrast low-IAT did not this significant effect (p>0.23). In NoGo condition high-IAT showed an increased brain response for videogames/gambling compared with neutral [respectively (F(1,27)=6.90, p=0.001, η2=0.30, F(1,27)=7.50, p=0.001, η2=0.34)] within the left frontal areas. In contrast in NoGo low-IAT showed an inverse trend: a decreased brain response for videogames/gambling compared to neutral stimuli [respectively (F(1,27)=7.16, p=0.001, η2=0.34, F(1,27)=8.88, p=0.001, η2=0.37)] indistinctly within the frontal left and right sides.

Fig. 3 Alpha power modulation as a function of stimulus type and Go/NoGo task for (a) high-IAT and (b) low-IAT. IAT, Internet Addiction Inventory.
sLORETA analysis
For alpha, a significant differential activation comparing Go/NoGo was found in the left frontal areas (t=5.68, p≤0.01; BA9 x=4, y=50, z=4), whereas the other areas did not reveal different profile as a function of Go/NoGo. In addition, higher-IAT showed a specific left frontal activation compared with low-IAT in response to more rewarding cues (for videogames t=8.13, p≤0.01; BA9 x=6, y=48, z=3, for gambling t=6.60, p≤0.01; BA9 x=7, y=52, z=2).
Regression analysis
Distinct multiple regression analyses were performed for each condition. Since sLORETA showed significant effect between conditions within the prefrontal cortex, regression analyses were performed only in this cortical area. Predictor variables were BAS/BAS-R subscales and predicted variables were frequency band in Go or NoGo condition for each stimulus category, from one hand; RTs variations on the other hand. This study reports the correlations between predictors and predicted variable (R), the explained variance (R 2), and the regression weights (β) for the regression equation.
About alpha, BAS and BAS-Reward measure accounted for alpha power in Go and NoGo for high-IAT, with reduced alpha values for gambling and videogames stimuli (Figs 4a and b). No other effect was statistically significant (Fig. 4b).

Fig. 4 Scatterplot of Behavioural Activation System (BAS) and BAS-Reward subscale (BAS-R) in relationship with Go/NoGo task and stimulus type for (a) response times (RTs) and (b) alpha band power.
About RTs, BAS and BAS-reward accounted for reduced RTs in high-IAT for both Go and NoGo condition. No other effect was statistically significant.
Discussion
The present research aimed to explore the contribution of rewarding bias in IA. IAT, alpha brain oscillations and BAS were used as integrated measures to test brain activity and behavioural response towards potential internet rewarding stimuli, such as gambling, videogames, compared with neutral cues when a Go/NoGo task was submitted. Lateralisation effect was also tested to support the hypothesis of an ‘unbalance’ effect between the left (more reward-related) and the right PFC.
Three main effects were found and they are discussed in the present section. First, IAT affected the response to more rewarding cues, with increased performance (reduced RTs). Specifically in both Go and NoGo condition high-IAT subjects revealed decreased RTs compared with low-IAT when they have to respond to rewarding cues. A similar profile was found for alpha modulation, that is an increased brain activity (less alpha) for more rewarding cues in high-IAT than low-IAT indistinctly for Go and NoGo condition. Moreover this effect was more left-lateralised, as reported by sLORETA analysis: an increased left frontal activity was observed for high-IAT in response to rewarding stimuli. Finally, BAS measure and reward-subscale were related to IAT, from one hand; they were predictive of RTs and alpha modulation as a function of high/low-IAT, from the other hand.
First a main effect was found in relationship with IAT construct, since subjects rated as high-IAT adopted a specific behaviour in response to Go-NoGo task in relationship with the stimulus category. Indeed they demonstrated to be more significantly responsive to potentially rewarding conditions, that is videogames and gambling cues, with a general higher performance. More generally the performance (RTs) was affected by both task condition and stimuli: indeed, whereas in general a reduction of RTs was revealed in response to Go than NoGo condition, videogames and gambling stimuli registered the lowest RTs values. In this case a sort of ‘facilitation effect’, with an increased performance for more salient stimuli, may be suggested. In addition an interesting and specific result was present in NoGo condition, which showed antithetic behaviour as a function of high- versus low-IAT profile: indeed whereas high-IAT maintained a reduction of RTs as shown in Go condition, low-IAT increased their RTs mainly for rewarding cues. The low-IAT behaviour was shown to be generally related to a cognitive cost due to the inhibitory mechanisms activated in order to control and inhibit the response. This cost was directly related to the increased necessity to modulate the subjective response when a more salient stimulus is processed (increased cost for videogames/gambling).
However, higher-IAT values predicted an opposite behaviour, with a similar performance (lower RTs than low-IAT subjects) even when the inhibitory mechanisms should be active (to control the NoGo response). That is, high-IAT subjects seem not to ‘pay’ for the inhibition process, with a sort of virtual gain induced by the high salience of the rewarding cues. These two contrasting effects (salience vs. inhibition) appear to argue in favour for salience related to rewarding contexts for high-IAT; in favour for inhibition mechanisms for low-IAT. Therefore, whereas the inhibitory mechanisms should have required more cognitive resources to control the explicit behaviour (with increased RTs) in lower-IAT subjects, the subjective performance may present a systematic ‘gain’ mainly for the most salient category (gambling cues) with a more ‘immediate’ and ‘impulsive’ response by high-IAT subjects.
A similar effect was found for alpha band modulation. Indeed subjects revealed a decreased alpha (higher brain response) in concomitance to more rewarding cues in Go condition. High-IAT showed this specific behaviour, with significant lateralised effect. Indeed a clear prefrontal left localisation was observed for these subjects when they had to respond to videogames or gambling stimuli. However, as shown for RTs, a divergent profile was found in NoGo condition when high-IAT versus low-IAT subjects were compared. Left PFC was indistinctly more activated in Go and NoGo condition for high-IAT, whereas low-IAT were subjected to inhibitory processes when NoGo task was performed: their brain activity was reduced, as signalled by alpha increasing, presumably to allow a functional inhibition of their response.
Indeed, as shown in previous research (Reference Yamanaka and Yamamoto60), NoGo condition is generally associated with the necessity to control the response and, therefore, the observed alpha modulation is consistent with previous observations with Go/NoGo task data (Reference Barry61–Reference Bernat, Nelson, Steele, Gehring and Patrick65) as well as other control-related processes such as response error and feedback processing (Reference Gehring and Willoughby66–Reference Palmero-Soler, Dolan, Hadamschek and Tass71). However in the present research a new and interesting result was that, for the first time, this decreased brain activity was mainly found in response to specific online task which used gambling and videogames stimuli. The significant impact of such categories may reveal the necessity for the subjects (mainly for low-IAT) to highly control and suppress their behaviour in response to specific and potentially ‘rewarding’ categories compared with the neutral ones. This result may also underline that NoGo condition and potentially ‘rewarding’ cues more consistently and directly activate the subjective resources finalised to ‘inhibit’ or suppress automatic responses. In contrast the potential response bias which affects the high-IAT behaviour limits consistently the impact of the inhibitory mechanisms, showing the prevalence of rewarding over inhibition.
Moreover, the contribution of both BAS/BIS and IAT measures was demonstrated in the online task. Indeed, as reported by regression analysis, they affected the subjects’ performance on both alpha brain oscillation and RTs levels. In fact BAS predicted the alpha band variations, although only for the sub-group of high-IAT. Indeed both the increased performance (higher RTs) and higher cortical responsiveness for rewarding contexts was predicted by BAS and BAS-R construct in the case of active response condition (Go) and in the case of inhibitory control condition (NoGo). That is, the motivational measure of BAS and the specific BAS-R subscale were able to describe a specific attitude and sensitiveness to the salience (in term of rewarding power) of the external context.
Taken together these facts suggest that higher-BAS and BAS-R subjects may present anomalies for some cognitive functions in governing inhibitory mechanisms, as well as dysfunctional frontal neural substrates that mediate these functions. Indeed they revealed an increased prefrontal responsiveness (Reference Hester, Murphy and Garavan72): this increased prefrontal activity in response to NoGo condition and to more rewarding cues (gambling and videogames) may be related to the inability to control the impulsive response.
Also impulsivity was previously reported as an explicative factor in IA. Specifically, neurocognitive models of addiction disorders often implicate impulsivity as a major component. However a second potential explanation of the present results is related to the significance of the stimuli category (gambling and videogames), that is the proper rewarding effect of such stimuli (Reference Kamarajan, Rangaswamy and Manz73). Indeed a significant finding of the present study is that higher BAS showed a significant PFC hyper-activation for ‘rewarding’ categories. (Reference Başar, Başar-Eroglu, Karakas and Schurmann74). Cortical oscillation variations has been shown to be related to a variety of motivational and emotional aspects of human behaviour, including reward processing (Reference Balconi, Finocchiaro and Canavesio14,Reference Balconi, Falbo and Brambilla18,Reference Kamarajan, Rangaswamy and Cholrian45,Reference Trujillo and Allen67,Reference Palmero-Soler, Dolan, Hadamschek and Tass71,Reference Basar and Guntekin75–Reference Luu, Tucker and Makeig80). In addition, prior findings, from both neuroimaging and electrophysiological studies, have reported dysfunctional neural reward systems in different forms of addictions (such as alcohol dependence) (Reference Camara, Rodriguez-Fornells and Munte81–Reference Wrase, Schlagenhauf and Kienast89). Overall, these topographic differences in addiction during reward processing may indicate a possible dysfunction in the neural reward circuitry. Diekhof et al. (Reference Diekhof, Falkai and Gruber90), have outlined the neural mechanisms underlying reward processing and decision-making processes in the healthy brain as well as pathophysiological alterations in the neural reward system observed in addictive and mood disorders. Integrating both dimensions as possible mechanism for addiction and drug-seeking behaviour, Schoenbaum et al. (Reference Schoenbaum, Roesch and Stalnaker91) reasoned that addicted individuals commonly exhibit a decreased ability to control the desire to obtain drugs (i.e. inadequate inhibitory control), despite knowledge about the aversive consequences following drug intake or the low expectation of actual pleasure expected from the drug (i.e. decision making and reward consequences). Therefore, higher BAS value may explain the deficits in impulse control and inhibitory mechanisms in terms of uncontrolled behaviour. A general rewarding bias may be added to explain this specific sensitivity to more rewarding conditions (gambling and videogames).
Therefore, in the light of earlier reports on reward processing in healthy subjects as well as in addition, hyperesponsivity in prefrontal areas in subjects higher in BAS and mainly BAS-R may suggest a dysfunctional reward circuitry, which might serve as a hallmark feature of future addictive behaviour. Source analysis pointed out that the cortical generators of these bands, and the localisation of the main modulation effect related to the Go/NoGo task, are frontally localised and mainly within the left DLPFC. Furthermore, because the cortical generators of alpha in response to rewarding stimuli were reported to be in the left frontal areas, alpha response can be attributed to an impairment in frontal lobe functioning.
Therefore, based on the present research, we hypothesise that inhibitory deficits and reward mechanisms observed in some subjects (higher-IAT and higher-BAS) may be due to the implication (and some anomalous activity) of frontal network system: since response inhibition is a function of frontal lobes (Reference Fuster92), the cortical hyper responsiveness in both Go and NoGo condition would imply a frontal lobe dysfunction in terms of processing of rewarding stimuli. A related aspect was the direct relationship existing between IAT and BAS constructs, that is they may be considered as related measures able to predict a potential addiction behaviour.
In synthesis, high sensitivity to IAT construct could be considered as a marker of dysfunctional reward processing and cognitive control. More generally a possible link among impulsivity, reward-related behaviour, and potential internet addiction may be suggested, specifically for higher IAT, BAS, and BAS-R (Reference Kamarajan, Rangaswamy and Tang93). In addition, our current study has demonstrated a basic relationship between left DLPFC alpha response, inhibitory control (NoGo), and rewarding cue sensitivity. However, a critical point is that it could be debated whether the anomalous cortical and behavioural response to gambling cues represents more a specific dysfunction in reward processing or a general deficiency in inhibitory control (Reference Kamarajan, Rangaswamy and Manz73). In any cases, we suggest that an integrated view, which includes brain oscillations in conjunction with Go/NoGo task and motivational measures (BIS/BAS measures), can potentially serve as a useful marker for differentiating the web addiction from the normal control and the highly rewarding cue responders from low-rewarding cue responders.
Some limitations should be reported in the present study. First of all the present research considered sub-clinical sample as indicated by IAT cut-off and it did not properly considered pathological subjects. Therefore ‘addiction vulnerability’ was explored instead of a pathological profile per se. For this reason, all the main points we discussed were related to specific IAT profiles (and scores), which include for the high-IAT a ‘moderate’ or ‘severe’ internet addiction level (as indicated by IAT cut-offs). Therefore the present results may suggest a sort of ‘vulnerability’ marker as suggested by both IAT and BAS measures, more than an overt pathological profile. In addition, other concurrent measures, that is neuroimaging acquisition, could better support the localisation effect that we found within the DLPFC in response to different experimental condition (i.e. GoNo task and IAT groups). Finally, from the point of view of the ecological validity, other stimulation condition should be included, which might allow to compare different types of stimuli more or less related to the gambling effect.
Acknowledgements
No specific to be added.
Authors’ contribution: Michela Balconi contributed to ideation and design, data analysis, drafting the paper, approval to final version of the paper. Roberta Finocchiaro contributed to design, data collection, revise the paper, approval to final version of the paper.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/neu.2016.9







