Significant outcomes
-
∙ A decrease in prefrontal kynurenic acid (KYNA) along with unchanged 3-hydroxykynurenine (3-HK) observed in the Flinders Sensitive Line (FSL) rats suggests that tryptophan metabolism is directed towards the microglial branch of the kynurenine pathway.
-
∙ Our results support the idea that aberrations in the kynurenine pathway participate in the pathophysiology of major depressive disorder (MDD).
Limitations
-
∙ Only one metabolite of the microglial branch of the kynurenine pathway was analysed.
-
∙ Further studies are needed to identify the mechanism(s) behind the presently shown imbalance of the kynurenine pathway.
Introduction
Depression is one of the leading causes of disability worldwide, however the underlying biochemical aberrations are essentially unknown. Although the traditional view regarding the pathophysiology of depression has focussed on perturbations in monoaminergic functions, in particular, serotonin neurotransmission, more recent findings point to an involvement also of glutamate signalling mechanisms. One of the most prominent findings in this regard come from clinical studies where N-methyl-d-aspartate (NMDA) receptor antagonists, principally ketamine, generate a robust antidepressant effect already within hours after administration (Reference Zarate, Singh and Carlson1–Reference Skolnick, Layer, Popik, Nowak, Paul and Trullas4). Further, a large body of genetic studies and cytokine analysis of serum or cerebrospinal fluid (CSF) in humans suggests a connection between immune activation and MDD (Reference Najjar, Pearlman, Alper, Najjar and Devinsky5). In that context, the tryptophan degradation along the kynurenine pathway may emerge as a possible candidate contributing to depression symptomatology. Thus, this route of tryptophan metabolism forms at least two neuroactive metabolites; KYNA, acting as an antagonist at the glycine site of the NMDA-receptor and at the cholinergic α7 nicotinic receptor, and quinolinic acid (QUIN), an NMDA-receptor agonist (Reference Schwarcz, Bruno, Muchowski and Wu6). The brain kynurenine pathway, which is critically regulated by cytokines of the innate immune system, consists of two branches, KYNA is formed in astrocytes, whereas 3-HK synthesis and further downstream metabolites, such as QUIN takes place in microglia (Reference Schwarcz, Bruno, Muchowski and Wu6). In the present study, two crucial metabolites of the brain kynurenine pathway, that is KYNA and 3-HK were analysed in FSL rat, an animal model of depression.
Material and methods
Animals
Female FSL and Flinders Resistant Line (FRL) rats (age 10–12 weeks) from the breeding colonies maintained at the Karolinska Institutet were used. Animals were maintained under standard laboratory conditions with free access to chow pellet and tap water in a light-controlled room (12 h light/dark cycle, light on at 6:00 a.m.), under constant temperature (22°C) and humidity (40–55%). In total, 10 FSL rats and 10 FRL rats were used in this study. As the day of the oestrus can affect the immunological and hormonal balance in the central nerves system (CNS), measures were taken (daily vaginal smear) to control for this variable (Reference Bogdanov, Bogdanova and Koulchitsky7–Reference Jiménez-Vasquez, Overstreet and Mathé8).
Brain sample preparation
Rats were guillotined, the brains taken out from the skull and immediately dissected on dry ice. A coronal slice, defined in this experiment as prefrontal cortex (PFC), was cut 2.4 mm (average wet weight 38 mg) from the tip of the frontal cortex (FC). Other brain areas were dissected according to the Glowinski and Iversen method (Reference Glowinski and Iversen9) into FC, hippocampus, striatum and cerebellum. Brain samples were frozen on dry ice and stored at −80°C; 0.4 M perchloric acid was added in an amount corresponding to three times the tissue weight and homogenised (Bullet Blender® Next Advance Inc. Averill Park, NY, USA). Homogenates were centrifuged at 14 000 rpm for 5 min and supernatants were diluted ×1.1 with 70% perchloric acid and stored at −20°C for subsequent analysis of KYNA and 3-HK. Measures were taken to allow a constant low temperature (22°C) throughout the handing of all samples.
Analysis of KYNA
KYNA was analysed with an isocratic reversed-phase high-performance liquid chromatography (HPLC) system, including a dual-piston, high-pressure liquid delivery pump Shimadzu LC-10AD (Shimadzu Corporation, Kyoto, Japan), a ReproSilPur C18 column (4×100 mm; Dr. Maisch GmbH, Ammerbuch, Germany) and a fluorescence detector (Jasco Ltd, Hachioji City, Japan) with an excitation wavelength of 344 nm and an emission wavelength of 398 nm (18 nm bandwidth). A mobile phase of 50 mM sodium acetate (pH 6.2, adjusted with acetic acid) and 7.0% acetonitrile was pumped through the reversed-phase column at a flow rate of 0.5 ml/min. Samples (50 μl) were manually injected by a Rheodyne® 7725i injector (IDEX, Oak Harbor, WA, USA) into a 100 μl loop. Zinc acetate (0.5M not pH adjusted) was delivered after the column at a flow rate of 10 ml/h by a peristaltic pump (P-500; Pharmacia, Uppsala, Sweden). Signals from the fluorescence detector were transferred to a computer for analysis using Datalys Azur software (Grenoble, France). The retention time of KYNA was about 7–8 min. Inter and intra coefficients of variation were 2.3% and 2.2%, respectively, for this assay. The sensitivity of the system was verified throughout the session by analysis of KYNA standards with concentrations ranging between 0.156–10 nM, which resulted in a linear standard plot.
Analysis of 3-HK
3-HK was analysed with an isocratic reversed-phase HPLC system, coupled to an electrochemical detector (Coulochem III; ESA Inc., Chelmsford, MA, USA), similar to what is previously described (Reference Agudelo, Femenía and Orhan10). A mobile phase consisting of 20 mM sodium phosphate, 0.7 mM octanesulfonic acid and 10% acetonitrile (pH set to 3.2 using acetic acid) was pumped through a ReproSil-Pur C18 column (4×150 mm, Dr. Maisch GmbH), at a flow rate of 0.6 ml/min, delivered by a LC-20AD VP HPLC pump (Shimadzu Corporation, Kyoto, Japan). Samples of 20 μl (kept at --25°C until analysis) were manually injected through a Rheodyne® 7725i injector (IDEX) into a 100 μl loop. The retention time of 3-HK was about 8.5 min. Signals from the detector were transferred to a computer for analysis with Clarity (DataApex Ltd, Prague, The Czech Republic). Inter and intra coefficients of variation were 2.7% and 2.1%, respectively. The limit of detection was at least 20 times lower than the reported values in this study. 3-HK was not detectable in two FC samples from FSL animals. Generally, values of 3-HK were similar to what is recently reported in controls rats by Schwarcz and co-workers (Reference Ceresoli-Borroni, Guidetti, Amori, Pellicciari and Schwarcz11–Reference Notarangelo, Wilson and Horning12).
Statistical analysis
The statistical software package GraphPad Prism® 6 (GraphPad Software Inc., San Diego, CA, USA) for Mac OS X was used. All data are expressed as median with interquartile range and analysed using the non-parametric Mann–Whitney U-test followed by Bonferroni correction for multiple comparisons. A p-value <0.05 was considered statistically significant throughout the study.
Results
KYNA levels in both FSL (n=10) and FRL rats (n=10) varied between different brain regions. In the PFC, KYNA levels were significantly lower in FSL compared with FRL animals (Table 1). However, KYNA levels did not differ between the two strains with regard to FC, striatum, hippocampus or cerebellum.
Table 1 Levels of kynurenic acid (KYNA) and 3-hydroxykynurenine (3-HK) and KYNA/3-HK ratio in different brain regions of Flinders Sensitive Line (FSL) and Flinders Resistant Line (FRL) rats
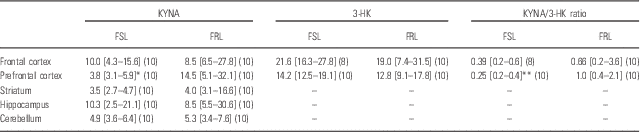
Median concentrations [interquartile range] of KYNA and 3-HK are expressed in nM. Numbers of animals are given in brackets. Differences in KYNA, 3-HK levels and KYNA/3-HK ratios between FSL and FRL rats were evaluated by Mann–Whitney U-test followed by the Bonferroni test for multiple comparison.
*p<0.05, **p<0.01.
Levels of 3-HK did not differentiate between FSL and FRL rats in either PFC or FC. However, in the PFC a significant reduction in the KYNA/3-HK ratio was found in FSL compared with FRL rats. No differences were found in other brain regions (Table 1).
Discussion
FSL, a genetic rat model of depression and their controls, FRL, have been a valuable choice to explore the pathophysiology of depression, effect of gene-environment interaction and effect of antidepressant treatment (Reference El Khoury, Gruber, Mørk and Mathé13–Reference Shrestha, Pine and Luckenbaugh18). Phenotypically, the FSL rats are similar to depressed patients and exhibit dysregulation of serotonin, glutamate and neuropeptideY neurotransmission (Reference Overstreet, Friedman, Mathé and Yadid19–Reference Ryan, Musazzi and Mallei20). In line with a recent study using an enzyme-based microelectrode array for glutamate detection (Reference Hascup, Hascup and Stephens21), the present study suggests that FSL rats display frontal glutamatergic overactivity. Thus, a reduced concentration of the NMDA-receptor antagonist KYNA in the PFC of the FSL rat would promote endogenous activation of the NMDA-receptor. This is also in line with a recent study showing that FSL rats exhibit increased glutamatergic neurotransmission in hippocampal CA1 area concomitant with reduced expression of the glial glutamate transporter (Reference Gómez-Galán, De Bundel, Van Eeckhaut, Smolders and Lindskog22).
The presently shown aberration of the kynurenine metabolism, that is reduced levels of KYNA in the PFC concomitant with apparently unchanged levels of 3-HK, indicate a disproportion between the two main branches of this pathway (Reference Schwarcz, Bruno, Muchowski and Wu6). This is reflected by the decrease in KYNA/3-HK ratio in PFC suggesting that tryptophan metabolism along the kynurenine pathway is directed towards the microglial, 3-HK and QUIN containing branch. In support of an imbalanced metabolism of the kynurenine pathway in MDD, it was recently shown that antidepressant drugs like fluoxetine, citalopram, amitriptyline and imipramine increase the KYNA/3-HK ratio in primary astroglial cultures (Reference Kocki, Wnuk, Kloc, Kocki, Owe-Larsson and Urbanska23). Moreover, physical exercise, generally known to induce antidepressant effects in humans (Reference Josefsson, Lindwall and Archer24) as well as in FSL rats (Reference Bjørnebekk, Mathé and Brené25), weaken the microglial branch of the kynurenine pathway (Reference Agudelo, Femenía and Orhan10). Further, in a cohort of suicidal attempters low CSF KYNA was associated with severe depressive symptoms (Reference Bay-Richter, Linderholm and Lim26).
The mechanism behind the reduced PFC KYNA in FSL rats is obscure. The specific reduction in KYNA concentration in the PFC in these rats is in line with a role of this area to regulate cognitive functions, planning and emotional behaviour (Reference Negrón-Oyarzo, Aboitiz and Fuentealba27), in contrast to the FC, which is partly dedicated to motor functions/locomotion. Indeed, imaging studies have consistently reported neurophysiological abnormalities of the PFC in MDD patients (Reference Johnstone, van Reekum, Urry, Kalin and Davidson28–Reference Dutta, McKie and Deakin30). Clearly, the presently observed specific reduction in PFC KYNA concentration in FSL rats may affect glutamatergic and gamma-aminobutyric acid (GABA)ergic signalling in this area (Reference Beggiato, Tanganelli, Fuxe, Antonelli, Schwarcz and Ferraro31). Thus, our results are in line with previous observation pointing to a role of PFC in major depression, although the precise mechanism behind this condition is obscure. The kynurenine pathway is critically regulated by cytokines (Reference Schwarcz, Bruno, Muchowski and Wu6). Thus, several enzymes of the kynurenine pathway are known to be induced by pro-inflammatory cytokines, most importantly tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase, both being rate-limiting enzymes of the kynurenine pathway (Reference Guillemin, Smith, Smythe, Armati and Brew32–Reference Sellgren, Kegel and Bergen33). Another enzyme of importance for the formation of KYNA is kynurenine-3-monooxygenase. An induction of this enzyme, for example, by interferon-gamma (Reference Parrott and O’Connor34) would promote the downstream metabolism of kynurenine to 3-HK and QUIN, in favour of a reduced astrocytic KYNA production. Although there is mounting evidence for a relationship between depression and neuroinflammation (Reference Furtado and Katzman35), information on immune activation in FSL rats is still sparse and somewhat conflicting. Thus, FSL rats show a number of peripheral immunological aberrations compared with FRL rats (Reference Friedman, Irwin and Overstreet36–Reference Carboni, Becchi and Piubelli38). Recently though, it was shown that the expression of immune-related genes like S100b and complement factor C3 is specifically down-regulated in several brain regions of FSL rats (Reference Strenn, Suchankova and Nilsson39). Further studies on cytokine regulation of the kynurenine pathway, as well as inflammatory mechanisms to account for behavioural and biochemical aberrations in FSL rats are necessary to identify the mechanism behind the presently shown imbalance of the kynurenine pathway.
In conclusion, FSL rats show an imbalance in the two main branches of the kynurenine pathway as reflected by a reduction in KYNA concentration in the PFC compared with FRL rats. This aberration, restored by antidepressants and physical exercise, may participate in the pathophysiology of MDD.
Acknowledgements
Supported by the Swedish Medical Research Council (grants no 2838, 10414, 2009-7052 and 2013-2838), the Swedish Brain Foundation, Åhlén-stiftelsen, Petrus och Augusta Hedlunds Stiftelse and the AstraZeneca-Karolinska Institutet Joint Research Program in Translational Science. No funding sources had any role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. Authors’ Contributions: A.A.M. bred the animals, harvested and dissected the brains. X-C.L. and M.G. performed the biochemical and statistical analyses, and participated in the writing of the manuscript. S.E., G.E. and A.A.M. conceived the hypothetical background and contributed to study design and writing.
Conflicts of Interest
The authors have no competing interests to declare. A.A.M. is an Associate Editor in Acta Neuropsychiatrica. However, A.A.M. did not handle the journals processing of the manuscript or was involved in any decisions related to the present work.
Ethical Standards
All experiments were approved by and performed in accordance with the guidelines from the Ethical Committee of Northern Stockholm, Sweden.




