Summations
• Evaluation of the quality of life (QoL) has acquired increasing importance in patients with degenerative diseases.
• The lack of treatment modalities for neurological disorders and disease progression emphasise the need for palliative therapies that have to be evaluated according to international guidelines.
• The development of the methodology for QoL evaluation continues towards more precise assessment using computer adaptive testing, implementation of electronic methods for data collection, integration of health measurement and patient preference weighting, rigorous statistical analysis and meaningful interpretation of QoL data.
Considerations
• Although several studies have shown the importance of QoL in the last few years, QoL scales are not used in clinical practice.
• This review describes the main QoL scales in neurological disorders, but many others have not been shown.
Introduction
Measurement of the QoL is becoming increasingly important in clinical patient management. The World Health Organization (WHO) has expanded and codified the definition of health to include mental and social well-being, making it multidimensional. This has permitted us, in the last few decades, to develop QoL concepts and adopt different instruments for multidimensional evaluation of health (Reference Perez, Huang and Jansky1). The main reason for the rapid development of QoL measures in healthcare is the growing recognition of the importance of understanding the impact of healthcare interventions on the patient's QoL rather than merely treating their bodies (Reference Welsh2). Further, physicians have always intended to find out and understand how their patients feel. This is particularly important for patients affected by neurodegenerative disorders, either disabling or life threatening (Reference Gregory, Johnston and Pratt3). Evidence that measuring QoL provides a better evaluation of these latter conditions is presented in the recent literature. The aim of this review was to assess the role and importance of principal QoL scales in neurodegenerative and demyelinating disorders such as Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS), which account for a significant and increasing proportion of morbidity and mortality in the developed world. Largely as a result of increased life expectancy and changing population demographics, neurodegenerative disorders are becoming increasingly common (Reference Staren, Gupta and Braun4). Evaluation of QoL has acquired increasing importance in neurodegenerative diseases. Treatment of neurodegenerative diseases places a substantial medical, social, and psychological burden on patients and their families and profoundly affects the QoL of all persons involved. QoL refers to people's emotional, social, and physical well-being and their ability to function in daily life (Reference Nakanishi, Hanihara, Mutai and Nakaaki5). QoL measures attempt to directly evaluate the impact of neurodegenerative diseases or interventions on people's ability to function in life. Besides this global conceptualisation of QoL, there is a growing field of research on QoL measures focussed on the measurement of health-related QoL (HRQoL). Instruments aimed at measuring the patient's health status outlook enable us to quantify the loss of QoL caused by disease and the improvement that can be achieved by interventions (Reference Jamroz-Wiśniewska, Stelmasiak and Bartosik-Psujek6). Disease-specific measurements are devised to assess the impact of a specific disease across a spectrum of important domains of life. They evaluate the relevant domains for a specific disease. Currently, most of the tools consider the physical conditions, the psychological comfort, and the level of activity, whereas a few consider, for example, the social sphere (Reference Riepe and Mittendorf7). For some tools an external evaluator is needed, for example, the physician, whereas in the majority of cases the questionnaires are answered by the patient himself. In patients suffering from neurodegenerative diseases (such as AD, PD, MS, and ALS), the evaluation of QoL could involve significant difficulties because of the relative unreliability of subjective assessment in the early stages of disease, as well as because of language barriers that make it impossible to obtain information about the patient's experience during later stages. During the course of the disease, every patient could reach a stage of cognitive decline in which any type of introspective evaluation of memory might be possible (8). Perez et al. argue that specific instruments tend to be more responsive to changes and more sensitive to discriminating the range of impairment because of their focus on the most relevant aspects of the QoL for the problems assessed. For all these reasons, it is necessary to study other fields on the basis of carefully selected specific measures of QoL chosen as being of particular importance to patients and to the hypotheses being tested. However, a critical analysis of the properties of the growing range of generic and disease-specific measures is necessary to guide and direct researchers and clinicians towards the most appropriate measures in terms of reliability, validity, and sensitivity to change instruments used in measuring the QoL (does it really measure what it is supposed to measure?); reliable (does it give the same measurement after repeated administrations in stable patients?); sensitive (does it reflect clinically meaningful differences in the QoL across the broad spectrum of clinical conditions?); and responsive (does it detect changes when the patient's condition changes?). In Italy at present some translated and validated tools are being increasingly used in the neurological field (Reference Small, Rabins, Barry and Buckholtz9). The aim of this work review was to assess the role of the principal QoL scales as a state-of-the-art measuring tool for assessing the QoL of patients suffering from neurodegenerative diseases.
Methods/tools
Alzheimer's Disease Questionnaire
The dementia QoL Instrument (DQoL) was specifically designed for self-assessment of QoL in AD patients with dementia using the Mini Mental State Examination (MMSE) ⩾ 12, without the intervention of the caregiver. The original work included a structured interview consisting of 96 items that investigated the characteristics of patients and their experiences of living with dementia. The interview included questions on the following: functional status, basic and advanced activities of daily living, mobility, physical well-being, social interaction skills, aesthetic awareness, and perception of the QoL. The instrument takes ∼10 min to administer. The validation work has led to the current version, which consists of 56 items divided into five domains (aesthetics, positive affection, negative affection, self-esteem, feeling of belonging) (Reference Brod, Stewart, Sands and Walton10) (see Table 1). Item-stems were made as simple as possible and a five-point visual scale was used to present multiple choice responses to patients. All points on the response scale are associated with verbal descriptors. Screening questions ensure that patients understand the instructions on the questionnaire and the response format for the scale. In a sample of 99 patients diagnosed with mild to moderate dementia (range from 12 to 21 MMSE), only 4% could not correctly answer the screening questions and thus were not administered the entire scale. For patients who completed the DQoL, internal consistency reliabilities for subscales were moderate to high. There were no significant differences between patient groups with mild and moderate dementia severity in terms of scale reliability (Reference Brod, Stewart, Sands and Walton10).
Table 1 Definition of the domains assessed in DQoL

DQoL, dementia quality of life.
Adapted from Brod et al. (Reference Brod, Stewart, Sands and Walton10).
QoL-Alzheimer's disease (QoL-AD) has been specifically constructed for self-assessment of QoL in patients suffering from dementia with MMSE > 12 without the intervention of the caregiver. The original instrument foresaw a structured interview composed of 96 items that investigated the characteristics of the patients and their experience of living with dementia; this interview included the evaluation of: the functional condition, basic and complex activities of daily life, mobility, physical comfort, and wellness in social life, ability to interact, aesthetic sense and perception of the quality of life. Validation has reduced the original version to 56 items divided into five domains (aesthetics, positive affections, negative affections, self-esteem, feeling of belonging) and took about 15–20 min to answer (Reference Logsdon, Gibbons, McCurry and Teri11). The authors propose the scale as a useful tool for the evaluation of the long-term effects of treatment (see Table 2). Although it is keenly recognised that there is no ‘gold standard’ or superior instrument for assessing QoL, this study has shown that both the QoL-AD and DQoL are useful instruments for capturing QoL from the perspective of the patient with dementia. However, given that the QoL-AD had better rates of completion and internal reliability in this study, the QoL-AD may be the most favourable instrument, particularly for those with more severe cognitive impairment and, to a less extent, functional impairment. Researchers, however, should consider the type of data that they require and for what purpose before making an informed decision on which instrument to employ. It may also be advisable to examine QoL using at least two measurements and to also consider collection of qualitative data as a complementary source of information to ensure the best assessment of QoL and capture of the true perspective of the person with dementia. Items for the QoL-AD were selected to reflect domains of QoL in older adults based on a literature review of QoL in geriatric populations. Face validity and comprehensiveness were ensured by having AD patients, caregivers, older adults without dementia and dementia experts review potential items (see Table 2). The final scale is composed of 13 items that measure the domains of physical condition, mood, memory, functional abilities, interpersonal relationships, ability to participate in meaningful activities and financial situation. Response options are four-point multiple choice options (1 = poor, 4 = excellent). Scale scores range from 13 to 52, with higher scores indicating greater QoL. Patients and caregivers typically complete the QoL-AD separately. Patients are interviewed and caregivers respond to the QoL-AD items on a questionnaire. Composite scores that combine reports from patients and caregivers are weighted to improve the patient's self-report. The scale takes an average of 10 min to administer to patients, and caregivers take <10 min to complete the questionnaire (Reference Logsdon, Gibbons, McCurry and Teri11). Psychometric properties of the QoL-AD were initially evaluated in a group of 77 AD outpatients and their caregivers (Reference Logsdon, Gibbons, McCurry and Teri11). A follow-up study with a larger sample of 177 AD patients was recently published (Reference Jenkinson, Fitzpatrick, Brennan, Bromberg and Swash12), in which 155 of the 177 patients interviewed were able to complete the QoL-AD. Mean MMSE for patients who did not complete the questionnaire was 4.1 compared with 18.1 for those who did (range 4–29); all patients with MMSE scores above 11 were able to complete the QoL-AD. In addition to greater cognitive impairment, patients who did not complete the questionnaire also showed significantly more impairment in basic and instrumental ADLs. Validity was indicated by low to moderate correlations between QoL scores and the MMSE and reports of instrumental activities of daily living, depression, and engagement in pleasant activities (Reference Jenkinson, Fitzpatrick, Brennan, Bromberg and Swash12). Validity of patient scores in the second study was indicated by correlations between QoL-AD scores and several measures of domains hypothesised to be associated with QoL: behavioural competence, psychological status, physical function and interpersonal environment. There were stronger associations between caregiver-reported QoL and measures of these other domains (Reference Logsdon, Gibbons, McCurry and Teri11).
Table 2 Item QoL-AD
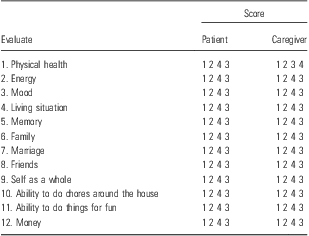
QoL-AD, quality of life-Alzheimer's disease.
Adapted from Logsdon et al. (Reference Logsdon, Gibbons, McCurry and Teri11).
The Apparent Affect Rating Scale (AARS) in projects of search is used to assess the QoL in patients institutionalised and affected by moderate to severe dementia. It includes five domains (three belonging to negative symptoms such as anger, anxiety, fear, depression and sadness and two to positive symptoms such as pleasure and interest). This scale requires the assistance of an evaluator trained to interpret the signs and facial expressions of the patient that imply emotions. The period of observation was 5 min. The evaluator should be empathetic and sensitive to nonverbal expressions and should have a good knowledge of the patients, their experiences and the environment in which the evaluation is being carried out (Reference Jenkinson, Fitzpatrick, Brennan, Bromberg and Swash12) (see Table 3). The authors of AARS have used different methods to implement the model. Some investigators have interpreted these five domains as defining features of QoL, whereas others have viewed some factors as predictors of QoL and others as indicators of QoL. In fact, some instruments incorporate items on functional and cognitive impairment in the scale, whereas others consider these factors as potential predictors of QoL but not as defining features. Some authors noted that items on cognition and physical functioning in QoL measures were included; there are many problems because these domains inevitably decline with advancing dementia (Reference Jenkinson, Fitzpatrick, Brennan, Bromberg and Swash12).
Table 3 Item dell'AARS
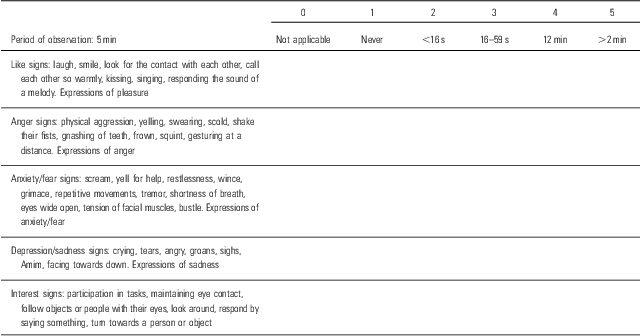
AARS, Apparent Affect Rating Scale.
Adapted from Lawton (Reference Lawton13).
Parkinson's Disease Questionnaire
Parkinson's Disease Questionnaire (PDQ-39) consists of 39 questions and the PDQ-8 (short form of the PDQ-39), PDQ-which analyse the subject in eight sizes for mobility, activities of daily life, stigma, social support, cognition, communication and physical discomfort. These tools help to collect information, combined with clinical data, and give an overview of the disease extended to the psychosocial consequences on the life of the subject, with implications on the choice of the most appropriate pharmacological interventions, either physical or psychological. The correlation of the PDQ-39 with scales that assess variables such as balance, posture and the fear of falling, as well as with the Unified Parkinson's Disease Rating Scale (UPDRS) or Hoehn and Yahr stage (H&Y), can provide more details about the phenomenon, revealing the incidence of the disorder, as well as describing the benefits and concerns while performing functional tasks and activities. The PDQ-39, a disease-specific 39-item QoL instrument for use in patients with PD, has been shown not only to have good reliability and validity but also to demonstrate consistent responsiveness and reproducibility. In addition to its impressive psychometrics, the PDQ-39 can be easily interpreted and provides the ability to assess the overall impact of the illness. Consequently, the PDQ-39 is the most widely used disease-specific patient-completed rating scale used in PD and has been widely used in many trials to assess the effectiveness of treatment. In addition, the NINDS in its NET-PD project is utilising the PDQ-39 to standardise outcome measures that are more inclusive in terms of QoL and nonmotor aspects of PD, compared with the UPDRS scale (NINDS: 2006 Parkinson's Disease Research Plan: 12–13). On the basis of these interviews and discussions with patients, items were created and a questionnaire was developed. The PDQ-39 has been used as an outcome measure in numerous clinical trials to test the effectiveness and clinical significance of many surgical, pharmacological and psychological treatments (Reference Jenkinson and Fitzpatrick14) (see Table 4). Several authors have shown that PDQ-39, and particularly its summary index (PDQ-39SI), is a widely used patient-reported clinical trial end point. A basic assumption when summing items into a total score is that they represent a common variable. The authors have assessed the unidimensionality of the PDQ-39SI using Rasch and confirmatory factor analysis. Both analyses showed model misfit. Adjustment for differential item functioning and disordered response category thresholds did not improve the model fit, and residual analyses showed deviation from unidimensionality. These data indicate multidimensionality and challenge the interpretation and validity of PDQ-39SI scores.
Table 4 Item PDQ-39

PDQ, Parkinson's Disease Questionnaire.
Adapted from Peto et al. (Reference Peto15).
Amyotrophic Lateral Sclerosis Questionnaire
The Amyotrophic Lateral Sclerosis Assessment Questionnaire (ALSAQ-40 and ALSAQ-5) contains 40 questions that measure five inherent areas pertaining to physical and mental health: ‘physical mobility’ (10 questions), ‘daily activity and independence’ (10 questions), ‘to eat and to drink’ (3 questions), ‘communication’ (7 questions) and ‘emotional operation’ (10 questions). The questions refer to the condition perceived by the patient during the last 2 weeks; the answers are quantified on a five-point Likert scale. The ALSAQ-5 contains five questions instead, each belonging to one of the five dimensions of the ALSAQ-40 (Reference Jenkinson, Fitzpatrick, Brennan, Bromberg and Swash12). The lack of treatments for ALS patients and the disease progression indicate the need for palliative therapies that have to be evaluated according to international guidelines. ALSAQ-40 and its shortened form, ALSAQ-5, are widely used ALS patient-focussed disease questionnaires (Reference Jenkinson and Fitzpatrick14). The purpose of the Jenkinson is to validate the Italian version of ALSAQ-40 and ALSAQ-5 in a large cohort of Italian ALS patients and to further characterise QoL in relation to muscle strength, motor disability and respiratory failure. The authors have recruited 76 ALS patients to complete the Italian version of ALSAQ-40 and ALSAQ-5. To verify the test–retest reliability, 30 patients were re-evaluated after 3 months. The internal reliability of the translated ALSAQ-40 scales and the test–retest reliability were assessed by means of a statistical index such as Cronbach's α. The SF-36 Questionnaire and Revised ALS Functional Rating (ALSFR-R) scale were used to assess the validity of the Italian ALSAQ-40 (Reference Ambs, Warren, Bellizzi, Topor, Haffer and Clauser16). The Medical Research Council (MRC) and Forced Vital Capacity (FVC) scores for limb muscles were used to measure the degree of patient loss of strength and respiratory failure, respectively. The Italian adaptation of the psychometric properties of ALSAQ-40 and ALSAQ-5 is reliable and similar to those of the original English version. The Italian adaptation showed a very good internal consistency (for all subscales Cronbach's α >0.86) and a good construct validity, as shown by the patterns of correlation between the subscales and SF-36 scores. ALSAQ-5 showed a positive correlation with the corresponding total and subscale scores of the ALSAQ-40 (Spearman's correlation >0.73). The authors have found a strong correlation between Italian ALSAQ-40 and ALSFRS-R scores and between MRC and FVC scores and Physical Mobility and ADL/Independence ALSAQ-40 subscale scores. The emotional functioning subscale on the ALSAQ-40 and either muscle strength or functional ability are not correlated. Emotive connotations that patients assign to their life can remain positive when their health is severely impaired. In conclusion, the authors have found the Italian adaptation of ALSAQ-40 and ALSAQ-5 questionnaires to be valid, reliable and useful as disease-specific QoL instruments for Italian ALS patients. The study by Palmieri and colleagues suggest that the QoL can be maintained as physical function declines (Tables 5 and 6).
Table 5 Item ALSAQ–40

ALSAQ, Amyotrophic Lateral Sclerosis Assessment Questionnaire
Adapted from Jenkinson et al. (Reference Jenkinson, Fitzpatrick, Brennan, Bromberg and Swash12).
Table 6 Item ALSAQ–5

ALSAQ, Assessment Questionnaire Lateral Sclerosis Assessment Questionnaire
Adapted from Jenkinson and Fitzpatrick (Reference Jenkinson and Fitzpatrick14).
Multiple Sclerosis Questionnaire
The Multiple Sclerosis QoL Health Survey (MSQOL-54) is a multidimensional HRQoL measure that combines both generic and MS-specific items into a single instrument. The developers utilised the SF-36 as the generic component to which 18 items were added to tap MS-specific issues such as fatigue, cognitive function, etc. This 54-item instrument generates 12 subscales along with two summary scores, as well as two additional single-item measures. The subscales are as follows: physical function, limitations in physical role, limitations in emotional role, pain, emotional well-being, energy, health perceptions, social function, cognitive function, health distress, overall QoL and sexual function. The single-item measures are satisfaction with sexual function and change in health. The MSQOL-54 is a structured, self-report questionnaire that the patient can generally complete with little or no assistance. It may also be administered by an interviewer. However, patients with visual or upper extremity impairments may need to have the MSQOL-54 administered as an interview. Interviewers should be trained in basic interviewing skills and in the use of this instrument (Reference Brazier, Roberts and Deverill17) (see Table 7). Several studies have demonstrated that problems other than physical disability, such as mental health problems and bladder and sexual problems, adversely affect the QoL of MS patients. New studies are also needed to further determine the factors that contribute to the reduced QoL of MS patients. Scientific evaluation of such interventions using QoL questionnaires as a measure of patients’ perspectives will allow the most useful strategies to be selected. Finally, published results are lacking from randomised clinical trials on the effect of interferon-β on the QoL. A study conducted by the authors has shown a modest effect; the rest were all small and used incomplete designs. A few significant findings might suggest no real effect on the patient's QoL or might be related to the insensitivity of the instrument used. This underlines the need for further studies on the responsiveness of the instruments used (Reference Brazier, Roberts and Deverill17).
Table 7 Item MSQOL-54

Conclusion
In the last decade the number of studies on QoL in patients affected by neurological disorders has increased exponentially. Several instruments have been developed, some of which are available in various languages, but the use of QoL as an outcome measure in clinical trials for the disease still has many shortcomings. It lacks a priori specification of how data are analysed and often lacks information about the mode of administration of the questionnaires (direct interview, telephone, self-administration, or other modes) and completeness of compilation. These problems, however, are only partly attributable to the shortcomings of the researcher or a member still not convinced with the current view of enhancing the patient-centred outcomes (Reference Hanmer, Hays and Fryback19). Most of the instruments used consist of a set of scales that can in turn be aggregated into a total score and/or a limited number of composite indices. This is particularly important when a tool is used for multidimensional QoL, as the possibility of incurring an error of the first type is increased if we apply the statistical comparisons on individual domains separately, especially if they are re-evaluated over time. The specification of a priori and time domains in which a difference is expected overcomes the problem of multiple comparisons. The results reported in the literature show that, although the development and validation of QoL questionnaires for demyelinating and neurodegenerative diseases have reached a satisfactory level, the consensus on which QoL instruments are preferred in clinical trials and interpretation of results should be the subjects of further research and investigation (Reference Pittock, Mayr and McClelland20). In summary, as a result of the achievements of the past two decades, nowadays we have many reliable and valid tools to evaluate the QoL of patients with neurological disorders. The methodology for assessment of QoL continues to develop towards a more precision evaluation through computer adaptive testing, implementation of electronic methods for data collection, integration of health pro le measurement and patient preference weighting, rigour statistical analysis and meaningful interpretation of QoL data. In parallel, we have observed increasing application of QoL instruments as outcome measures in clinical trials and growing interest in their use to aid patient–clinician interaction and policy decision making. The scientific rigour of QOL research will determine the extent to which the resulting data are accepted by clinicians, policymakers and the public.









