Significant outcomes
• Bupropion increased the activity of the mitochondrial respiratory chain complex II and SDH.
• The present findings reinforce the hypothesis that antidepressants modulate brain energy metabolism.
Limitation
• The effect of bupropion may not be the same if we administrate the drug for a longer time.
• The effect of bupropion in an animal model of depression should also be studied.
Introduction
Depressive disorders, including major depression, are serious and disabling disorders often manifested with psychological, behavioural and physiological symptoms (Reference Rex, Schickert and Fink1–Reference Shelton3). Moreover, depression seems to have genetic backgrounds, also suggesting a biologic contribution to its origin. However, the exact pathophysiology of depression is not clearly understood (Reference Kessler, Gonagle and Zhao4).
Depression represents a major public health problem due to its high prevalence and psychosocial impact and significant societal costs (Reference Murray, Murray and Lopez2,Reference Kessler, Rubinow, Holmes, Abelson and Zhao5). In its report ‘The Global Burden of Disease', the World Health Organisation reported that unipolar major depression was the leading cause of disability in 1990, contributing to 10.7% of the total disability at ages 15–44 years (Reference Murray, Murray and Lopez2).
Antidepressants are very effective agents for preventing and treating depression and have been used clinically for more than 50 years. The monoaminergic theory on aetiology of the depressive disorder states advocates that the underlying cause of the disease is the insufficient noradrenergic (NA), serotoninergic (5-HT) and dopaminergic (DO) transmission in the central nervous system (CNS) (Reference Rex, Schickert and Fink1,Reference Murray, Murray and Lopez2,Reference Kessler, Rubinow, Holmes, Abelson and Zhao5,Reference Longone, Rupprecht, Manieri, Bernardi, Romeo and Pasini6). The therapeutic effect is slow and this action could be due to the indirect regulation of other neuronal signal transduction systems or the regulation of gene transcription following chronic treatment.
Bupropion is an atypical antidepressant (Reference Mico, Ardid, Berrocoso and Eschalier7) that acts via dual inhibition of norepinephrin and dopamine (DA) reuptake and is devoid of clinically significant serotonergic effects or direct effect of postsynaptic receptors. However, the affinity of bupropion for these two reuptake transporters is lower than most antidepressants. Thus, it is possible that bupropion may modulate other neurotransmitters in addition to monoamines (Reference Stahl, Pradko, Haight, Modell, Rockett and Learned-Coughlin8).
The antidepressants have contributed to the improvement of the depressive patients' quality of life and to make the treatment of depression more satisfactory (Reference Duman, Nakagawa and Malberg9,Reference Jacobs, Praag and Gage10). Several studies link brain energy metabolism impairment and animal models of psychiatric disorders (Reference Heales, Bolaños, Stewart, Brookes, Land and Clark11–Reference Madrigal, Olivenza and Moro14).
Brain is a tissue with high energy demands, so it contains a large number of mitochondria to obtain energy through oxidative phosphorylation (Reference Boekema and Braun15). Krebs' cycle is the major final common pathway for oxidation of carbohydrates, lipids and some amino acids. This metabolic pathway is located in the mitochondrial matrix and produces reducing equivalents in the form of nicotinamide adenine dinucleotide and flavin adenine dinucleotide that result in the production of large amounts of adenosine triphosphate (ATP) via oxidative phosphorylation (Reference Kelly, Gordon and Alpers16). Citrate synthase, malate dehydrogenase and succinate dehydrogenase (SDH) are enzymes of Krebs' cycle. The SDH is one of the most reliable markers of the mitochondrial capability to supply an adequate amount of ATP, as it is part of both the Krebs' cycle and the respiratory chain (complex II) (Reference Corrêa, Amboni and Assis17).
Mitochondrial respiratory chain is located in a special structure of the inner mitochondrial membrane. In most organisms, the mitochondrial respiratory chain is composed of four complexes (Reference Boekema and Braun15). It is well described that mitochondrial dysfunction has been implicated in the pathogenesis of a number of diseases affecting the brain (Reference Tyler18–Reference Moreira, Santos, Seiça and Oliveira24).
Another enzyme that is involved in energy production is creatine kinase (CK), it catalyses the reversible transphosphorylation of creatine by ATP (Reference Bessman and Carpenter25,Reference Wallimann, Wyss, Brdiczka, Nicolay and Eppenberger26). It was recently showed that CK is inhibited in animal models of neuropsychiatry disorders, such as bipolar disorder and after electroconvulsive shock (Reference Streck, Amboni and Scaini27,Reference Burigo, Roza and Bassani28).
Based on the hypothesis that energy impairment may be involved in the pathophysiology of depression and that some antidepressants may improve energy metabolism (Reference Kanarick, Matrov, Kõiv, Eller, Tõnissaar and Harro29,Reference Stanyer, Jorgensen, Hori, Clark and Heales30), in this present study we evaluated the activities of enzymes citrate synthase, malate dehydrogenase, SDH, complexes I, II, II-III, IV of mitochondrial respiratory chain and CK in brain of rats submitted to chronic administration of bupropion.
Materials and methods
Animals
Adult male Wistar rats (250–300 g) obtained from the Central Animal House of Universidade do Extremo Sul Catarinense were caged in groups of two with free access to food and water and maintained on a 12-h light-dark cycle (lights on 07:00 h) at a temperature of 22° ± 1°C. All experimental procedures were carried out in accordance with the Brazilian Society for Neuroscience and Behavior (SBNeC) recommendations for animal care, with the approval of local Ethics Committee.
Chronic administration of bupropion
Animals received daily administration of bupropion dissolved in saline (10 mg/kg, intraperitoneal) at 1 ml/kg body weight. The rats received injection once daily for 14 days. Control rats received an equivalent volume of saline, 1 ml/kg (intraperitoneal), for the same treatment period. Twelve hours after injection (Reference Kitamira, Fujitani and Kitagawa31), the rats were killed by decapitation and cerebral structures were rapidly removed and kept on an ice-plate.
Tissue and homogenate preparation
Prefrontal cortex, posterior cortex, hippocampus, striatum, cerebellum and hypothalamus were homogenised (1:10, w/v) in SETH buffer, pH 7.4 (250 mM sucrose, 2 mM EDTA, 10 mM Trizma base, 50 IU/ml heparin). The homogenates were centrifuged at 800 ×g for 10 min at 4 °C and the supernatants kept at −70 °C until being used for enzymes activity determination. The maximal period between homogenate preparation and enzyme analysis was always less than 5 days. Protein content was determined by the method described by Lowry et al. (Reference Lowry, Rosebough and Farr32) using bovine serum albumin as standard.
Activities of enzymes of Krebs' cycle
Citrate synthase activity. Citrate synthase activity was assayed according to the method described by Shepherd and Garland (Reference Shepherd and Garland33). The reaction mixture contained 100 mM Tris, pH 8.0, 100 mM acetyl CoA, 100 mM 5,5′-di-thiobis-(2-nitrobenzoic acid), 0.1% triton X-100, and 2–4 µg supernatant protein and was initiated with 100 µM oxaloacetate and monitored at 412 nm for 3 min at 25 °C.
Malate dehydrogenase activity. Malate dehydrogenase was measured as described by Kitto (Reference Kitto34). Aliquots (20 mg protein) were transferred into a medium containing 10 mM rotenone, 0.2% Triton X-100, 0.15 mM β-Nicotinamide adenine dinucleotide hydrate (NADH), and 100 mM potassium phosphate buffer, pH 7.4, at 37 °C. The reaction was started by addition of 0.33 mM oxaloacetate. Absorbance was monitored as described above.
Succinate dehydrogenase activity. Succinate dehydrogenase activity (SDH) was determined according to the method of Fischer et al. (Reference Fischer, Ruitenbeek and Berden35), measured by following the decrease in absorbance due to the reduction of 2,6-dichloroindophenol (2,6-DCIP) at 600 nm with 700 nm as reference wavelength (ε = 19.1/mM/cm) in the presence of phenazine methasulphate (PMS). The reaction mixture consisting of 40 mM potassium phosphate, pH 7.4, 16 mM succinate and 8 µM 2,6-DCIP was preincubated with 40–80 µg homogenate protein at 30 °C for 20 min. Subsequently, 4 mM sodium azide, 7 µM rotenone and 40 µM 2,6-DCIP were added and the reaction was initiated by addition of 1 mM PMS and was monitored for 5 min.
Activities of mitochondrial respiratory chain enzymes
Complex I activity. NADH dehydrogenase (complex I) was evaluated according to Cassina and Radi (Reference Cassina and Radi36) by the determination of the rate of NADH-dependent ferricyanide reduction at λ = 420 nm.
Complex II activity. The activities of succinate-2,6-DCIP oxidoreductase (complex II) was determined by the method described by Fischer et al. (Reference Fischer, Ruitenbeek and Berden35). Complex II activity was measured by following the decrease in absorbance due to the reduction of 2,6-DCIP at λ = 600 nm.
Complex II-III activity. The activity of succinate : cytochrome c oxidoreductase (complex III) was determined by the method described by Fischer et al. (Reference Fischer, Ruitenbeek and Berden35). Complex II-III activity was measured by cytochrome c reduction using succinate as substrate at λ = 550 nm.
Complex IV activity. The activity of cytochrome c oxidase (complex IV) was assayed according to the method described by Rustin et al. (Reference Rustin, Chretien and Bourgeron37), measured by following the decrease in absorbance due to the oxidation of previously reduced cytochrome c (prepared by reduction of cytochrome with NaBH4 and HCl) at λ = 550 nm with 580 nm as the reference wavelength (ε = 19.1/mM/cm). The activities of the mitochondrial respiratory chain complexes were calculated as nmol/min × mg protein.
Activity of CK enzyme
CK activity was measured in brain homogenates pretreated with 0.625 mM lauryl maltoside. The reaction mixture consisted of 60 mM Tris-HCl, pH 7.5, containing 7 mM phosphocreatine, 9 mM MgSO4 and approximately 0.4–1.2 µg protein in a final volume of 100 µl. After 15 min of pre-incubation at 37 °C, the reaction was started by the addition of 3.2 mmol of adenosine 5′-diphosphate (ADP) plus 0.8 mmol of reduced glutathione. The reaction was stopped after 10 min by the addition of 1 µmol of p-hydroxymercuribenzoic acid. The creatine formed was estimated according to the colorimetric method of Hughes (Reference Hughes38). The colour was developed by the addition of 100 µl 2% α-naphtol and 100 µl 0.05% diacetyl in a final volume of 1 ml and read spectrophotometrically after 20 min at 540 nm. Results were expressed as units/min × mg protein.
Statistical analysis
Data were analysed by one-way analysis of variance (ANOVA) followed by the Student's t-test when F was significant and are expressed as mean ± SD. All analyses were performed using the Statistical Package for the Social Science (SPSS) software.
Results
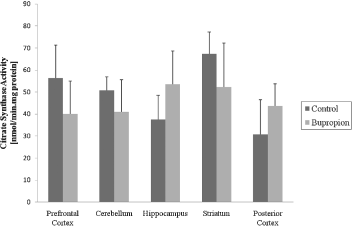
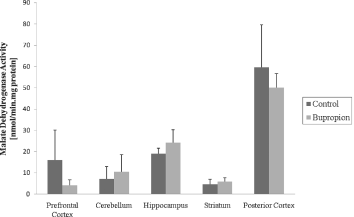
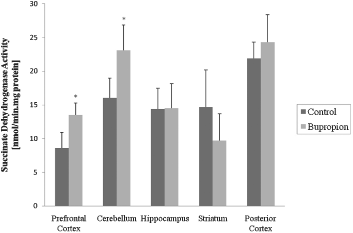
In the present work, we evaluated activity of some enzymes of Krebs' cycle, mitochondrial respiratory chain and CK in brain of rat after chronic administration of bupropion. Our results showed that the citrate synthase and malate dehydrogenase activity were not altered in the structure's studies (Figs 1 and 2, respectively). SDH activity was increased in the prefrontal cortex and cerebellum after chronic administration of bupropion, but this enzyme activity was not altered in the hippocampus, striatum and posterior cortex (Fig. 3).
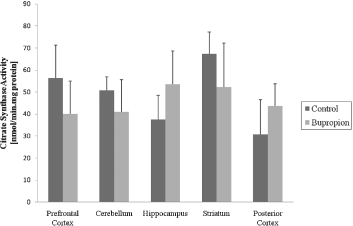
Fig. 1. Citrate synthase activity after chronic administration of bupropion in the prefrontal cortex, cerebellum, hippocampus, striatum and posterior cortex of rats. Data were analysed by one-way analysis of variance (ANOVA) followed by the Student's t-test when F was significant. Values are expressed as nmol/min × mg protein, mean ± SD (n = 6).
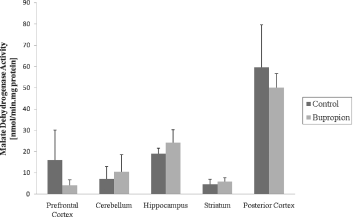
Fig. 2. Malate dehydrogenase activity after chronic administration of bupropion in the prefrontal cortex, cerebellum, hippocampus, striatum and posterior cortex of rats. Data were analysed by one-way analysis of variance (ANOVA) followed by the Student's t-test when F was significant. Values are expressed as nmol/min × mg protein, mean ± SD (n = 6).
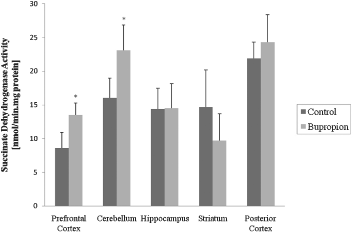
Fig. 3. Succinate dehydrogenase activity after chronic administration of bupropion in the prefrontal cortex, cerebellum, hippocampus, striatum and posterior cortex of rats. Data were analysed by one-way analysis of variance (ANOVA) followed by the Student's t-test when F was significant. Values are expressed as nmol/min × mg protein, mean ± SD (n = 6). * Different from control; p < 0.05.
Complexes I, II-III and IV activities (Fig. 4a, c and d, respectively) were not altered by the chronic administration of bupropion in the structures studies. However, in the hippocampus and striatum the activity of complex II was increased, but in the prefrontal cortex, cerebellum and posterior cortex were not altered (Reference Kessler, Gonagle and ZhaoFig. 4b). Finally, the activity of CK was not altered in the prefrontal cortex, cerebellum, hippocampus, striatum and posterior cortex (Fig. 5).

Fig. 4. Complex I activity (a), complex II activity (b), complex II-III activity (c) and complex IV activity (d) activity after chronic administration of bupropion in the prefrontal cortex, cerebellum, hippocampus, striatum and cerebral cortex of rats. Data were analysed by one-way analysis of variance (ANOVA) followed by the Student's t-test when F was significant. Values are expressed as nmol/min × mg protein, mean ± SD (n = 6). * Different from control; p < 0.05.

Fig. 5. Creatine kinase activity after chronic administration of bupropion in the prefrontal cortex, cerebellum, hippocampus, striatum and cerebral cortex of rats. Data were analysed by one-way analysis of variance (ANOVA) followed by the Student's t-test when F was significant. Values are expressed as nmol/min × mg protein, mean ± SD (n = 6).
Discussion
Mitochondria are responsible for several essential processes in the development and normal function of the body, including energy production, apoptosis, and the generation of reactive oxygen species. Krebs' cycle is the major final common pathway for oxidation of carbohydrates, lipids and some amino acids, hence factors that influence this cycle flux will be of critical importance for mitochondrial oxidative phosphorylation that is the major ATP-producing pathway, which supplies more than 95% of the total energy requirement in the cells (Reference Tyler18). In the present work, we observed that the activities of citrate synthase and malate dehydrogenase enzymes of Krebs' cycle, complexes I, II-III and IV of mithocondrial respiratory chain and CK were no altered after chronic administration of bupropion in rats. However, SDH activity in the prefrontal cortex, and cerebellum while in cerebellum and hippocampus complex II activity was increased after chronic administration of bupropion.
Decrease in mitochondrial ATP production rates may cause oxidative stress by excessive formation of reactive species, which leads to oxidative damage to proteins, lipids and deoxyribonucleic acid (Reference Arnaiz, Coronel and Boveris39). Besides, reactive oxygen species inhibit the mitochondrial respiratory chain, resulting in the generation of more reactive species, forming a cyclic phenomenon (Reference Adam-Vizi40,Reference Assis, Rezin and Comim41).
Damage to the mitochondrial electron transport chain has been suggested to be an important factor in the pathogenesis of a range of psychiatric disorders (Reference Kanarick, Matrov, Kõiv, Eller, Tõnissaar and Harro29–Reference Kitamira, Fujitani and Kitagawa31) including major depression (Reference Scaini, Santos and Benedet42–Reference Rezin, Cardoso and Gonçalves45). Gardner et al. (Reference Gardner, Johansson and Wibom43) showed a significant decrease in mitochondrial ATP production rates and mitochondrial enzyme ratios in muscles of major depressive disorder patients. Madrigal et al. (Reference Madrigal, Olivenza and Moro44) also reported that complexes I-III and II-III of mitochondrial respiratory chain were inhibited in rat brain after chronic stress (immobilisation for 6 h during 21 days). Rezin et al. (Reference Rezin, Cardoso and Gonçalves45) also demonstrated that mitochondrial respiratory chain is inhibited in brain of rats after chronic variable stress (40 days), suggesting that energy metabolism impairment may occur in depressive disorders. Considering that metabolism impairment is probably involved in the pathophysiology of depressive disorders, the modulation of energy metabolism by antidepressants could be an important mechanism of action of these drugs.
It is known that DA and norepinephrine (NE) are involved in the regulation of energy homeostasis, owing to an additive or synergistic effect on these two systems (Reference Wellman46). In this context, bupropion inhibits reuptake of the catecholamines DA and NE through blockade of the DA reuptake transporters (DAT) and NE reuptake transporters (NET) (Reference Ascher, Cole and Colin47,Reference Stahl, Pradko, Haight, Modell, Rockett and Learned-Coughlin48) causing a modulation on the activity of this system. In our study, we found that SDH activity was increased in the prefrontal cortex and cerebellum and in the hippocampus and striatum the activity of complex II was increased after chronic administration of bupropion, showing a specific affinity of this complex, considering that SDH is an important enzyme to complex II.
Most antidepressants need chronic administration before achieving clinical effects; the mechanisms involved in this delay are not known, but their therapeutic efficacy is probably mediated by long-term molecular adaptations. In this context, several studies demonstrated that mitochondrial respiratory chain enzymes are activated in the brain of adult rats after chronic administration of paroxetine, nortriptyline and venlafaxine (Reference Scaini, Maggi and De-Nês49). Studies also demonstrated that citrate synthase, SDH and CK are increased by chronic administration of paroxetine in brain of adults' rats (Reference Scaini, Santos and Benedet42,Reference Santos, Scaini and Rezin50).
In the study of Assis et al. (Reference Assis, Rezin and Comim41) was showed that the imipramine increased CK activity in the cerebellum and prefrontal cortex. On the other hand, Agostinho et al. (Reference Agostinho, Scaini and Ferreira51) showed that the inhibitory effect of olanzapine and fluxetine on CK activity in the brain of rats leads us to speculate whether these drugs impair energy metabolism.
Based on the hypothesis that metabolism impairment might be involved in the pathophysiology of depression and according to some previous work that has demonstrated that some antidepressants may improve energy metabolism (Reference Kanarick, Matrov, Kõiv, Eller, Tõnissaar and Harro29,Reference Stanyer, Jorgensen, Hori, Clark and Heales30), we demonstrated that the bupropion is in accordance with other authors who have shown that some enzymes of energy metabolism are activated by chronic administration of some antidepressants. Finally, even though it is difficult to extrapolate our findings to the human condition, it may be suggested that some antidepressants modulate brain energy metabolism.
Acknowledgements
This work was supported by grants from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC) and Universidade do Extremo Sul Catarinense (UNESC).