Significant outcomes
• Intra-nucleus accumbens (NAc) administration of an exchange protein directly activated by cAMP (Epac)-selective activator impaired amphetamine-induced conditioned place preference (CPP).
• Intra-NAc administration of Epac and protein kinase (PKA) activator also impaired amphetamine-induced CPP.
Limitations
• Lack of Epac-selective inhibitors created a limitation at the time of the present study.
Introduction
Reward-related learning occurs when previously neutral stimuli acquire an enhanced ability to elicit approach and other responses (Reference Beninger and Gerdjikov1). CPP relies on this type of learning. Dopamine (DA) D1-like (Reference Abrahams, Rutherford, Mallet and Beninger2) or D2-like receptor agonists produce a CPP (Reference Hoffman, Dickson and Beninger3), and D1-like or D2-like receptor antagonists block CPP when co-injected with a DA neurotransmission-enhancing agent such as amphetamine (Reference Hoffman and Beninger4). CPP produced by amphetamine relies on DA neurotransmission in the nucleus accumbens (NAc) (Reference Carr and White5). D1-like receptors are coupled to Gs proteins and their activation increases the activity of adenylate cyclase (AC) and 3′,5′-cyclic adenosine monophosphate (cAMP) and D2-like receptors are coupled to Gi proteins and inhibit AC-cAMP signaling.
cAMP acts through two different signalling pathways, one involving cAMP-dependent PKA and leading to activation of the transcription factor cAMP response element-binding protein (CREB) (Reference Konradi, Cole, Heckers and Hyman6) and the other involving exchange protein directly activated by cAMP (Epac) (Reference Kawasaki, Springett and Mochizuki7). Two isoforms of Epac, Epac1 and Epac2, have been discovered (Reference Breckler, Berthouze, Laurent, Crozatier, Morel and Lezoualc'h8). Activation of the Epac signalling pathway has been linked to depression, memory disorders, diabetes, cancer, heart failure, and inflammation (Reference Breckler, Berthouze, Laurent, Crozatier, Morel and Lezoualc'h8). cAMP-mediated effects by PKA and Epac are often associated with the same biological process in which they have either synergistic or opposite effects. For example, cAMP, through combined actions on PKA and Epac, influences the nuclear/cytoplasmic trafficking of DNA-dependent PKA (Reference Huston, Lynch and Mohamed9). On the other hand, increased activity of epithelial sodium channels produced by DA is completely mediated by the Epac and not the PKA pathway (Reference Helms, Chen, Ramosevac, Eaton and Jain10).
Sp-adenosine 3′,5′-cyclic monophosphorothioate triethylamanine (Sp-cAMPS) activates both PKA and Epac (Reference McKnight11,Reference Rehmann, Schwede, Døskeland, Wittinghofer and Bos12). Injections of Sp-cAMPS into the NAc inhibit the acquisition of CPP based on amphetamine (Reference Beninger, Nakonechny and Savina13). This may come about through occlusion of the reward-related signal (Reference Beninger and Gerdjikov1). PKA can be activated by Gs-coupled DA, serotonin (5-HT4), adenosine (A2) or N-methyl-d-aspartate (NMDA) glutamate receptors. Epac can similarly be activated by stimulation of noradrenaline, acetylcholine, corticotropin-releasing factor, adenosine (A2B), 5-HT7 or D1 receptors (Reference Lin, Johnson-Farley, Lubinsky and Cowen14–Reference Woolfrey, Srivastava and Photowala17). Thus, indiscriminate activation of PKA and Epac can turn on signals of other receptors and result in the masking of DA-mediated reward signals that seem to be necessary for the acquisition of reward-related learning.
The purpose of the present study was to test the hypothesis that the cAMP–Epac pathway may be involved in the acquisition of amphetamine CPP. Thus, we compared the effects of Epac-selective activation by 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (8-pCPT) (Reference Enserink, Christensen and de Rooij18) with those of co-activation of PKA and Epac by Sp-cAMPS on the acquisition of amphetamine CPP. Drugs were bilaterally injected into the NAc.
Materials and methods
Subjects
Wistar rats (Charles River, St. Constant QC) weighing 160–180 g upon arrival were pair-housed and kept on a 12-h reversed light–dark cycle (lights on at 1900 h) in a ventilated and temperature-controlled room (19–21°C; humidity 40–70%). Experiments were conducted during the dark phase. Rats had access to food and water at all times. The treatment of the rats adhered to the guidelines of the Canadian Council on Animal Care; Queen's University Animal Care Committee approved the protocol.
Surgery
Using standard stereotaxic surgical methods, guide cannulae made with 23-G (0.64 mm diameter) needles were bilaterally implanted into the NAc with level skull coordinates: 1.6 mm anterior to bregma, 1.4 mm lateral to the midline and 6.8 mm ventral from the skull surface.
Intra-NAc drug infusion
A pair of 10 μl microsyringes (Hamilton, Reno, NV, USA) was mounted on a microinfusion pump (KD Scientific, Holliston, MA, USA). Two injectors, made with metal tubing (0.31 mm diameter), bent at ∼30° so that they extended 1.2 mm beyond the guide cannulae, were inserted into polyethylene tubing (0.75 mm diameter). Injections of 0.5 μl were made into the NAc on each side over 30 s. Injectors were left in place for an additional 30 s. The rat was then immediately placed inside a compartment of the CPP box for conditioning (see below).
Drugs
Dextro-amphetamine sulphate (USP, Rockville, MD, USA), 8-pCPT (Biolog Life Science Inst., San Diego, CA, USA), and Sp-cAMPS (Sigma-Aldrich, Oakville, ON, USA) were dissolved in distilled water and stored in plastic tubes at −20°C.
Apparatus
Four boxes made of wooden sides and a Plexiglas cover with circular ventilation holes consisted of two compartments (27 × 38 × 34 cm high) joined by a tunnel (8 × 8 × 8 cm). The floor of one compartment was galvanised wire mesh (1 cm2) and the other of a stainless steel grid (1 cm apart). The walls were Plexiglas-covered; one was black and white alternating vertical stripes (1 cm) and the other urethane-sealed wood. The paradigm was unbiased. The tunnel could be closed with two guillotine-style Plexiglas doors. Tunnel floors were galvanised sheet metal. Two infrared emitters and detectors in each compartment and two in the tunnel detected the location of the animals and a computer calculated the time spent in each location and the level of activity (counts/30 min). Each box was placed in a styrofoam-insulated enclosure that was ventilated with an electric fan. A 7.5 W incandescent bulb installed inside the enclosure indirectly illuminated the box.
Procedure
There were 12 sessions (1/day) in three phases: pre-conditioning, conditioning and test. During pre-conditioning (three 15-min sessions), rats were placed in the box with an open tunnel; time spent in each compartments and in the tunnel was measured. During conditioning (eight 30-min sessions), rats received an injection of either the drug or vehicle (distilled water) and were immediately placed into a compartment with the tunnel closed. On days 1, 3, 5 and 7, the rats received the drug injection. On days 2, 4, 6 and 8, the rats received the vehicle injection. Activity counts were measured. During the test (one 15-min session) the rats were placed in the box with an open tunnel and the time spent in each compartment and in the tunnel was measured. A CPP was established if there was a significant increase in the time spent in the drug-paired compartment from pre-conditioning (averaged over 3 days) to testing.
A total of 16 groups were tested (n = 153). Animals were randomly assigned to groups. For 8-pCPT, independent groups received doses of 0 (20.0 μg/0.5 μl/side amphetamine alone) or amphetamine plus 0.73, 1.27, 1.45, 2.89, 5.78, or 11.56 μg/0.5 μl/side 8-pCPT; two additional groups were tested with amphetamine plus the 1.45 μg dose. For Sp-cAMPS, independent groups received doses of 0 (amphetamine alone) or amphetamine plus 0.1, 1.0, 10, 15, or 20 μg/0.5 μl/side Sp-cAMPS or 15 μg/0.5 μl/side Sp-cAMPS alone. The doses of the 8-pCPT and Sp-cAMPS were selected on the basis of the study by Ma et al. (Reference Ma, Abel and Hernandez19).
Histology
Formalin-fixed brains were sliced into 40-μm sections, mounted on gelatin-coated microscope slides and stained with cresyl violet. Hits and misses were judged by an observer who was blind to the CPP results of individual animals. Animals with cannulae outside of the NAc were excluded from the analysis.
Data analysis
For analysis of possible side bias during pre-conditioning, and for possible changes in tunnel time from pre-exposure to test for each experiment, paired sample t-tests were carried out.
For each of the 8-pCPT and Sp-cAMPS dose–response experiments, two-variable (group × phase) mixed analyses of variance (ANOVA) with independent groups and repeated measures on phase (pre-conditioning days averaged vs. test) determined the establishment of CPP. Where appropriate, a significant main effect was followed by Tukey's honestly significant difference (HSD) post-hoc analysis. A significant interaction was followed by tests of simple effects. In addition, planned t-tests compared the time spent in the drug-paired side during pre-conditioning and testing in each group to determine whether CPP had occurred.
Three-variable (group × treatment × day) mixed design ANOVA with independent groups and repeated measures on treatment (drug vs. vehicle) and day (1, 2, 3, 4) analysed locomotor activity during the conditioning sessions for 8-pCPT and Sp-cAMPS groups. Where appropriate, a significant main effect was followed by Tukey's HSD post-hoc analysis. A significant interaction was followed by tests of simple effects, which in turn were followed by Tukey's HSD pairwise comparisons as appropriate. Significance levels were set at p < 0.05.
Results
Side bias and tunnel time
In each experiment, a t-test of a possible side bias during the pre-conditioning phase revealed no significant effect (data not shown). T-tests also compared the tunnel time between pre-conditioning and testing. A significant decrease would increase chamber time and could inflate a change in time from pre-conditioning to test leading to a false positive CPP effect. No significant differences were found except when the three independent groups that received amphetamine plus 8-pCPT (1.45 μg/0.5 μl/side) were combined [amphetamine plus 8-pCPT (1.45 μg)†]. For this group, mean (±SEM) tunnel time (s) decreased from pre-conditioning (55.12±3.87) to test (46.50±3.89), t(27) = 2.26, p = 0.03. However, as this group failed to acquire CPP, the significant tunnel time difference was not relevant.
CPP
The amphetamine alone and amphetamine plus 8-pCPT groups (0.73, 1.27, 2.89, 5.78, and 11.56 μg) showed an increase in time spent on the drug-paired side from pre-conditioning to testing (Fig. 1, top). Groups treated with amphetamine plus 8-pCPT (1.45 μg) failed to show a similar change. ANOVA revealed a main effect of phase (pre-conditioning vs. testing) when all groups were combined, F(1, 92) = 22.61, p ⩽ 0.001. The average (±SEM) time (s) spent in the drug-paired compartment during testing (464.73±10.62) was significantly higher than the average time spent in the same compartment (averaged over 3 days) during pre-conditioning (414.84±6.60). The main effect of group and the interaction were not significant.

Fig. 1 Mean (±SEM) time (s) spent in the drug-paired compartment during pre-conditioning (mean over 3 days) and testing. Asterisk (*) above the bar indicates a significant difference from pre-conditioning in the same experiment based on paired samples t-test (p < 0.05). The ‘1.45†’ represents the data combined from three groups that received amphetamine (20 μg/0.5 μl/side) plus 8-pCPT (1.45 μg/0.5 μl/side). The ‘15 alone’ represents the group that received Sp-adenosine 3′,5′-cyclic monophosphorothioate triethylamanine (Sp-cAMPS) (15 μg/0.5 μl/side) alone without amphetamine. The amphetamine alone (20 μg/0.5 μl/side) groups shown (i.e., 8-pCPT dose of 0 μg and Sp-cAMPS dose of 0 μg) are identical. All doses are in μg/0.5 μl/side.
Planned t-tests revealed that treatment with amphetamine alone produced a CPP, t(14) = 2.75, p = 0.016. The tests further revealed that adding 8-pCPT (0.73, 1.27, 2.89, 5.78, 11.56 μg) to amphetamine did not affect the acquisition of a CPP, t(18) = 2.56, p = 0.02, t(8) = 2.51, p = 0.037, t(11) = 3.01, p = 0.01, t(6) = 6.56, p = 0.001, t(10) = 2.55, p = 0.029, respectively, but the addition of 8-pCPT (1.45 μg) inhibited the acquisition of a CPP. Three independent groups received amphetamine plus 1.45 μg 8-pCPT, two of which were tested to replicate the result from the first group. Each of the three groups on its own, t(8) = 0.36, p = 0.73, t(9) = −0.91, p = 0.39, and t(8) = 0.54, p = 0.60, respectively, and the groups combined failed to acquire a CPP, t(27) = 0.16, p = 0.87.
The groups treated with amphetamine alone or amphetamine plus Sp-cAMPS (0.1, 1.0, 10 μg) showed increased time spent in the drug-paired side from pre-conditioning to test (Fig. 1, bottom). Groups treated with amphetamine plus Sp-cAMPS (15, 20 μg) or Sp-cAMPS (15 μg) alone showed little change or a decrease. ANOVA revealed a main effect of group, F(6, 60) = 2.28, p = 0.047, phase, F(1, 60) = 10.95, p = 0.002, and a group × phase interaction, F(6, 60) = 3.79, p = 0.003. Tests of simple effects of phase (pre-conditioning vs. testing) for each group revealed that rats receiving amphetamine alone or amphetamine plus Sp-cAMPS (1.0, 10 μg) acquired a CPP, t(14) = 2.75, p = 0.016, t(7) = 3.89, p = 0.006 and t(7) = 2.58, p = 0.036, respectively, but those that received amphetamine plus Sp-cAMPS (15, 20 μg) failed to acquire a CPP, t(7) = 0.70, p = 0.51 and t(10) = −1.77, p = 0.11, respectively. Amphetamine plus Sp-cAMPS (0.1 μg) did not produce a significant CPP, but the effect approached significance, t(9) = 2.00, p = 0.077. Sp-cAMPS (15 μg) alone did not produce a significant effect.
Unconditioned activity
As in the case with the CPP analysis, the groups that received amphetamine plus 8-pCPT (all doses) were compared, whereas those that received amphetamine plus Sp-cAMPS (all doses) were compared. A significant main effect of treatment was observed in all groups. Thus, the injection of amphetamine plus 8-pCPT (all doses), amphetamine plus Sp-cAMPS (all doses), or Sp-cAMPS (15 μg) alone produced higher levels of locomotor activity compared with the injection of vehicle during conditioning (Fig. 2).

Fig. 2 Mean (±SEM) locomotor activity counts/30-min conditioning session on drug and saline vehicle days. Data are organised into drug and vehicle sessions, 4 days each, for each group. The ‘1.45†’ represents the data combined from three groups that received amphetamine (20 μg/0.5 μl/side) plus 8-pCPT (1.45 μg/0.5 μl/side). The ‘15 alone’ represents the group that received Sp-adenosine 3′,5′-cyclic monophosphorothioate triethylamanine (Sp-cAMPS) (15 μg/0.5 μl/side) alone, that is, no amphetamine was injected. The amphetamine alone (20 μg/0.5 μl/side) groups shown (i.e., 8-pCPT dose of 0 μg and Sp-cAMPS dose of 0 μg) are identical. All doses are in μg/0.5 μl/side.
Notably, the hyperactivity produced by the drug treatment was also seen in those groups that failed to acquire a CPP. This suggested that the failure to observe acquisition of a CPP by these groups was not due to an effect of the drug on motor stimulation produced by amphetamine.
Amphetamine plus 8-pCPT
ANOVA revealed a main effect of group, F(8, 92) = 2.66, p = 0.011; treatment, F(1, 92) = 153.92, p ⩽ 0.001, day, F(3, 276) = 2.64, p = 0.05; and a group × treatment interaction, F(8, 92) = 2.04, p = 0.05.
The group × treatment interaction reveals that some of the groups showed larger treatment effects than others. However, one-way ANOVA for simple effects of treatment (drug vs. vehicle) for each group revealed a significant effect in every case. One-way ANOVA for simple effects of group during drug treatment (collapsed over days) and during vehicle treatment (collapsed over days) revealed that drug treatments produced significantly different levels of locomotor activity between groups, F(8, 92) = 2.61, p = 0.01, whereas vehicle treatments did not (although the effect approached significance), F(8, 92) = 1.96, p = 0.06, providing the source of the group × treatment interaction.
Tukey's HSD post-hoc pairwise comparisons further revealed that the locomotor activity produced by drug treatment was significantly higher in the group that received amphetamine plus 8-pCPT (2.89 μg) than two of the three groups that received amphetamine plus 8-pCPT (1.45 μg)–amphetamine plus 8-pCPT [1.45 μg (second)], p = 0.01 and amphetamine plus 8-pCPT [1.45 μg (third)], p = 0.029.
Amphetamine plus Sp-cAMPS
ANOVA revealed a main effect of group, F(6, 60) = 5.42, p < 0.001, treatment, F(1, 60) = 160.60, p < 0.001, and a group × treatment interaction, F(6, 60) = 4.38, p = 0.001. It also revealed a main effect of day, F(3, 180) = 3.67, p = 0.013, a group × day interaction, F(18, 180) = 1.95, p = 0.015, a treatment × day interaction, F(2.52, 151.07) = 9.41, p < 0.001, and a group × treatment × day interaction, F(18, 180) = 2.62, p = 0.001. The group × treatment interaction reveals that some of the groups showed larger treatment effects than others. However, one-way ANOVA for simple effects of treatment for each group revealed a significant treatment effect in every case.
A test of simple interaction effects revealed that treatment × day interaction effects differed depending on the group, providing the source of the group × treatment × day interaction. Thus, the group that received amphetamine plus Sp-cAMPS (20 μg), F(3, 30) = 9.23, p < 0.001, and that received Sp-cAMPS (15 μg) alone, F(3, 18) = 4.23, p = 0.02, showed significant treatment × day interactions, whereas other groups did not. Generally, the treatment × day interaction reflected increasing differences between drug and saline effects from day 1 to days 2, 3, and/or 4.
Histology
For all experiments combined, 31 animals were excluded because of inaccurate cannulae placements on one or both sides. A total of 153 rats were found to have bilateral cannulae placements in the NAc and completed testing (Table 1).
Table 1 Breakdown of animal numbers for each group (hits) based on the histological examination of the brains
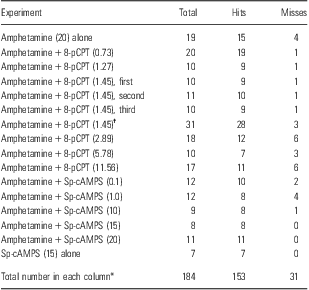
Sp-cAMPS, Sp-adenosine 3′,5′-cyclic monophosphorothioate triethylamanine.
Misses indicates rats with one or both cannulae outside of the nucleus accumbens.
†Represents combined values for the first, second, and third replication of this group.
*Numbers from the amphetamine + 8-pCPT (1.45)† group are excluded in these totals to avoid counting them twice.
Seizures
Treatment with amphetamine plus Sp-cAMPS (15 μg), amphetamine plus Sp-cAMPS (20 μg), or Sp-cAMPS (15 μg) alone produced seizures in some rats. Rats that had seizures and those that were suspected of having had seizures from their diminished mobility were removed from the experiment. The results of the present study are based on data that include only those rats that were seizure-free.
Discussion
There are a number of possible ways in which PKA and Epac may be involved in the acquisition of reward-related learning. Thus, PKA signalling alone may mediate reward-related learning, the Epac pathway alone may mediate this type of learning, or activation of both PKA and Epac signalling may be necessary.
In the present study, the Epac-selective activator 8-pCPT and the Epac and PKA activator Sp-cAMPS both inhibited the acquisition of amphetamine CPP. The finding with Sp-cAMPS is consistent with the observation of a disruption of reward-related learning with the cAMP analogues, Sp-cAMPS, or Rp-cAMPS, across different paradigms including CPP (Reference Beninger, Nakonechny and Savina13), appetitive approach conditioning (Reference Jentsch, Olausson, Nestler and Taylor20), and lever pressing for food (Reference Baldwin, Sadeghian, Holahan and Kelley21).
PKA and Epac in reward-related learning
The finding that selective activation of Epac by 8-pCPT (1.45 μg/0.5 μl/side) impaired the acquisition of amphetamine CPP implicates Epac in the acquisition of reward-related learning. This rules out the first possibility mentioned above, but more evidence is required to decide between the remaining two. Epac2 is involved in D1 receptor-mediated synapse remodelling and depression in cultured rat cortical neurons (Reference Woolfrey, Srivastava and Photowala17), further implicating Epac in DA functions. Ouyang et al. (Reference Ouyang, Zhang, Zhu, Schwede and Thomas22) showed that stimulation of PKA and Epac together in the hippocampus is required for contextual fear memory retrieval and that activation of Epac alone produced no rescue of retrieval (except at the highest dose of 2 μg) in memory-impaired mutant DA β-hydroxylase knockout mice. These results support an interactive effect of PKA and Epac in some psychological processes.
The contrasting dose–response curves of 8-pCPT and Sp-cAMPS suggest that Epac relays reward-related signals differently from PKA. The physiologically relevant cAMP affinity of Epac1 and the PKA holoenzyme may be quite similar (Reference Dao, Teigen and Kopperud23). These authors suggest that closeness to cellular compartments with locally increased cAMP (Reference Baillie and Houslay24) or the kinase substrate availability (Reference Viste, Kopperud, Christensen and Døskeland25) may decide whether Epac or PKA is activated first in response to a moderate cAMP increase in the intact cell. Some results suggest differential activation of the two cAMP signals. In neuroendocrine PC12 cells, activation of PKA alone led to cell proliferation; however, activation of both PKA and Epac led to neurite outgrowth (Reference Kiermayer, Biondi and Imig26). Results also suggested that PKA-dependent extracellular signal-regulated kinase (ERK) 1/2 was activated for a longer duration by Epac activation and that the sustained activity of ERK 1/2 changed the cAMP signal from a cell proliferative to a (anti-proliferative) neurite outgrowth signal. Garay et al. (Reference Garay, D'Angelo and Park27) reported that PKA had a stimulatory effect on dendritic cell maturation, Epac plus PKA suppressed dendritic cell maturation, and Epac signalling alone had no effect. In the present study, activation of the PKA pathway might have mediated the acquisition of reward-related learning, whereas activation of both PKA and Epac pathways might have produced a different biological signal and inhibited the acquisition of CPP.
Dose–response curve of 8-pCPT
The dose–response pattern of 8-pCPT was markedly different from that of Sp-cAMPS. Whereas Sp-cAMPS inhibited CPP at higher doses, but not at lower doses (except for 0.1 μg/0.5 μl/side, which failed to produce a significant CPP with p = 0.077), 8-pCPT inhibited CPP at only a middle dose (1.45 μg/0.5 μl/side). Ma et al. (Reference Ma, Abel and Hernandez19) showed that activation of Epac by 8-pCPT-29-O-Me-cAMP at a low dose (0.5 μg) enhanced memory in mice but at a higher dose (5 μg) produced no effect. Ma et al. (Reference Ma, Abel and Hernandez19) also reported that activation of PKA by Sp-cAMPS enhanced memory. If very low doses of 8-pCPT-29-O-Me-cAMP also had no effect, the dose–response curve of Ma et al. (Reference Baldwin, Sadeghian, Holahan and Kelley21) would resemble that produced by 8-pCPT on CPP in the current study.
A similar dose–response relationship has been observed in another CPP study (Reference McKnight11). CPP based on quinpirole, a D2-like receptor agonist, was inhibited by a D2-like receptor antagonist, metoclopramide, at a middle dose (10 mg/kg, i.p.), but not at a lower (1.0 mg/kg) or higher dose (20 mg/kg). Thus, the dose–response curve of 8-pCPT from the present study resembles the dose–response curve of the D2-like receptor antagonists on their effects on CPP. Perhaps Epac activation inhibits the acquisition of CPP based on amphetamine by a similar mechanism to that of a D2-like receptor antagonist, inhibiting the acquisition of CPP based on a D2-like receptor agonist.
Another possible explanation for the unusual dose–response curve for the action of 8-pCPT on amphetamine CPP is that higher concentrations of 8-pCPT (>1.45 μg/0.5 μl/side) inhibited phosphodiesterase1 (PDE1), PDE2, and PDE6 and that this effect masked the main effect of 8-pCPT (Reference Poppe, Rybalkin and Rehmann28). Finally, the possibility of the biological activity of 8-pCPT metabolites leading to non-specific effects is a concern. In this regard, PKA- and Epac-independent effects of 8-pCPT metabolites on ACTH-stimulated cortisol synthesis have been reported (Reference Enyeart and Enyeart29). Therefore, 8-pCPT metabolites possibly through unknown mechanisms may interfere with its main effects.
Conclusions
The objective of the present study was to investigate whether Epac, an intracellular signalling molecule targeted by the second messenger cAMP, may be involved in the acquisition of amphetamine CPP. To this end, a drug that stimulates PKA and Epac, Sp-cAMPS, or a drug that selectively activates Epac, 8-pCPT, was co-injected with amphetamine into NAc to observe its effect on the acquisition of CPP. Results provided some evidence that both Sp-cAMPS and 8-pCPT inhibited the acquisition of amphetamine CPP. The pattern of dose–response for the two activators, however, was considerably different. The results suggested that Epac may be involved in some aspect of reward-related learning. Epac may mediate the reward-related signal independently or in conjunction with PKA. The availability of a selective antagonist of Epac signalling is eagerly awaited and further studies are necessary to examine different possible roles of Epac in reward-related learning.
Acknowledgements
Supported by a grant from the Natural Sciences and Engineering Research Council of Canada to R.J.B. An abstract based on a portion of the data was published at the 36th annual meeting of the Society for Neuroscience, Atlanta, GA in 2006.




